Role of Circular RNAs in Cancer Immune Checkpoint Therapy: Focus on Anti-PD-1 Treatment
Abstract
Beginning with an overview of circRNA biogenesis and functionalities, we examine circRNA-mediated mechanisms that impact the PD-1 pathway in cancers such as colorectal, gastric, pancreatic, hepatocellular, bladder, breast, lung, and metastatic melanoma. CircRNAs exhibit diverse functions, including acting as microRNA (miRNA) and protein sponges, translational modulators, and gene expression regulators, thereby intricately modulating the efficacy of PD-1 blockade therapy. Despite remarkable advancements, the field necessitates further investigation to unravel the full spectrum of circRNA-PD-1 interactions and translate these findings into clinical practice effectively. Our review underscores the significant translational potential of circRNAs in cancer immunotherapy, highlighting their multifaceted role in shaping innovative therapeutic strategies. These circRNAs offer insights into the molecular mechanisms of malignancies and hold promise as reliable biomarkers for early detection, prognosis, and monitoring of treatment responses. By integrating circRNA profiling into clinical practice, we envision the development of more personalized treatment approaches that cater to the unique molecular and immune landscapes of individual patients, ultimately revolutionizing cancer management and improving patient outcomes.
Keywords:
Circular, RNA programmed, cell, death, 1 immune, checkpointsHighlights
Circular RNAs (circRNAs) have emerged as critical regulators of immune evasion in cancer by modulating programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) signaling pathways.
Recent studies demonstrate that specific circRNAs can directly or indirectly influence PD-1 expression, affecting T cell exhaustion and immune checkpoint blockade efficacy.
Circular RNA-mediated competitive endogenous RNA (ceRNA) networks represent promising therapeutic targets to enhance the response to PD-1 inhibitors.
Major unanswered questions include the precise mechanisms by which circRNAs modulate immune cell phenotypes within the tumor microenvironment and their clinical utility as predictive biomarkers.
Future perspectives suggest integrating circRNA profiles into immunotherapy decision algorithms and exploring circRNA-based therapeutics in combination with immune checkpoint blockade.
Introduction
Immune Checkpoints and Anti-PD-1 Therapy
Immunotherapies refer to the usage of stimulative or suppressive substances in order to change the outcomes of immune system interactions and antagonize the disease progression (1). In cancer immunotherapies, it is provided by cancer vaccines, adoptive cell therapies with tumor-infiltrating lymphocytes, chimeric antigen receptor (CAR) T-cell therapies, and immune checkpoint blockade.
Immune checkpoints (ICPs) typically function to suppress overactivated cell-mediated immune responses, thereby maintaining homeostasis, particularly normal anti- tumor immunity; concurrently, they prevent peripheral tissue damage (2). Examples of immune checkpoint proteins are programmed cell death 1 (PD-1), programmed cell death ligand 1 (PD-L1), cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4), lymphocyte activation gene 3 (LAG3), T-cell immunoglobulin (Ig) and mucin domain (TIM3), and V-domain Ig suppressor of T-cell activation (VISTA) (3). However, cancer cells acquired the ability to activate these ICPs to evade immune surveillance. For this reason, potential chemopreventive strategies should be developed to counteract the diminished or decreased immune cell activity in cancer treatment by ICP inhibitors (4). Immune checkpoint inhibitors (ICIs) represent a transformative therapeutic strategy designed to reverse this tumor-induced immune suppression (5). The core concept behind ICIs is to release the “brakes” placed on the immune system by the malignant cells, thereby restoring the effector T-cell capacity to recognize and eliminate the tumor (6). This novel mechanism of action has led to remarkable clinical success and significant improvements in patient outcomes across numerous solid tumors (7).
Nevertheless, the therapeutic utility of ICIs remains challenged by considerable patient response variability, the development of acquired resistance, and the potential for immune-related adverse events, necessitating ongoing research into predictive biomarkers and rational combination strategies (8,9).
Programmed cell death 1, which is also depicted as CD279, is an example of the immune checkpoint and can be found on the surfaces of activated T cells, B cells, dendritic cells (DCs), regulatory T cells (Tregs), and tumor-associated macrophages (TAMs) (2). Structurally, PD-1 protein contains a monomeric extracellular domain (ECD), transmembrane domain (TMD), and intracellular domain (ICD). In the tumor microenvironment (TME), T cell and PD-1 interacts with PD-L1 expressed in tumor cells (10). After the PD-1/PD-L1 interaction, immunoreceptor tyrosine-based inhibitory (ITIM) or switch motifs (ITSM) are activated, and later this interaction suppresses downstream T-cell receptor (TCR) signaling pathways and therefore inhibits T cell proliferation and the production of cytokines like interferon-gamma (IFN-γ) and interleukin-2 (IL-2) (11). In this way, tumor cells make the immune cells vulnerable while performing their functions (12). Recent research has uncovered that non-coding RNAs, especially circular RNAs (circRNAs), may play a significant role in influencing immune checkpoint pathways like PD-1, providing new opportunities for therapeutic interventions.
Biogenesis and Functions of circular RNAs
After the discovery of its role in viral replication in RNA viruses by Sanger et al. (13) in 1976, circRNAs were found to have distinct features, particularly in their unique structure among non-coding RNAs (ncRNAs), and are responsible for a vast number of both physiological and pathophysiological events, as discussed in detail elsewhere (14). Biogenesis of these special ncRNAs is dependent on the circularization of linear sequences of both exons and/or introns throughout the interaction with 5’-cap and 3’-poly-A tail, which are covalently bound and do not have the site for binding the RNA exonucleases, which drives downregulation of RNA components in the environment (15). After their biogenesis, circRNAs act like sponges to certain microRNAs (miRNAs), which are other ncRNAs that have diverse functions either inhibiting or activating the downstream effect of miRNA-associated pathways. It has been identified as a key regulator of gene expression pathways, functioning through interactions with enhancer and silencer proteins, mediating RNA splicing to generate diverse transcript variants, and contributing to protein translation processes (16). Although they are synthesized in the nucleus, they transfer to the cytoplasm and then eventually can be found in different body sites and cellular components such as exosomes, plasma, and interstitial fluids (17).
Circular RNAs in Tumorigenesis and Cancer Progression
Circular RNAs are emerging as critical regulators of immune cell function, influencing processes such as activ ation, differentiation, and immune evasion, particularly within the TME (18,19). By acting as sponges for specific miRNAs, circRNAs modulate gene expression in immune cells, shaping their activity and interactions. In T cells, circRNAs like circNCOA3 and circFGFR1 suppress cytotoxic responses by indirectly promoting the recruitment of immunosuppressive cells or reducing CD8+ T cells infiltration into tumors. Similarly, circRNAs regulate DCs by affecting their antigen-presenting capacity and cytokine production, as seen with circDLG1, which recruits myeloid-derived suppressor cells (MDSCs) and impairs T cell activation (20-22). These effects collectively hinder effective anti-tumor immunity, making circRNAs key players in immune regulation. In addition to their roles in T cells and DCs, circRNAs significantly influence macrophages, particularly TAMs, by driving their polarization toward an immunosuppressive M2 phenotype (23-25). For instance, circPRDM4 enhances this polarization via the hypoxia-inducible factor-1α (HIF-1α) pathway, supporting tumor immune evasion (26)
Circular RNAs also regulate other immune cells, such as Tregs, where they contribute to maintaining immune suppression in pathological conditions (27). In acute respiratory distress syndrome, circFLNA increases PD-1 expression on Tregs, promoting their anti-inflammatory functions (27,28). These findings underscore the diverse and interconnected roles of circRNAs across immune cell populations, highlighting their potential as targets for therapeutic intervention in diseases like cancer and inflammatory disorders (29-31).
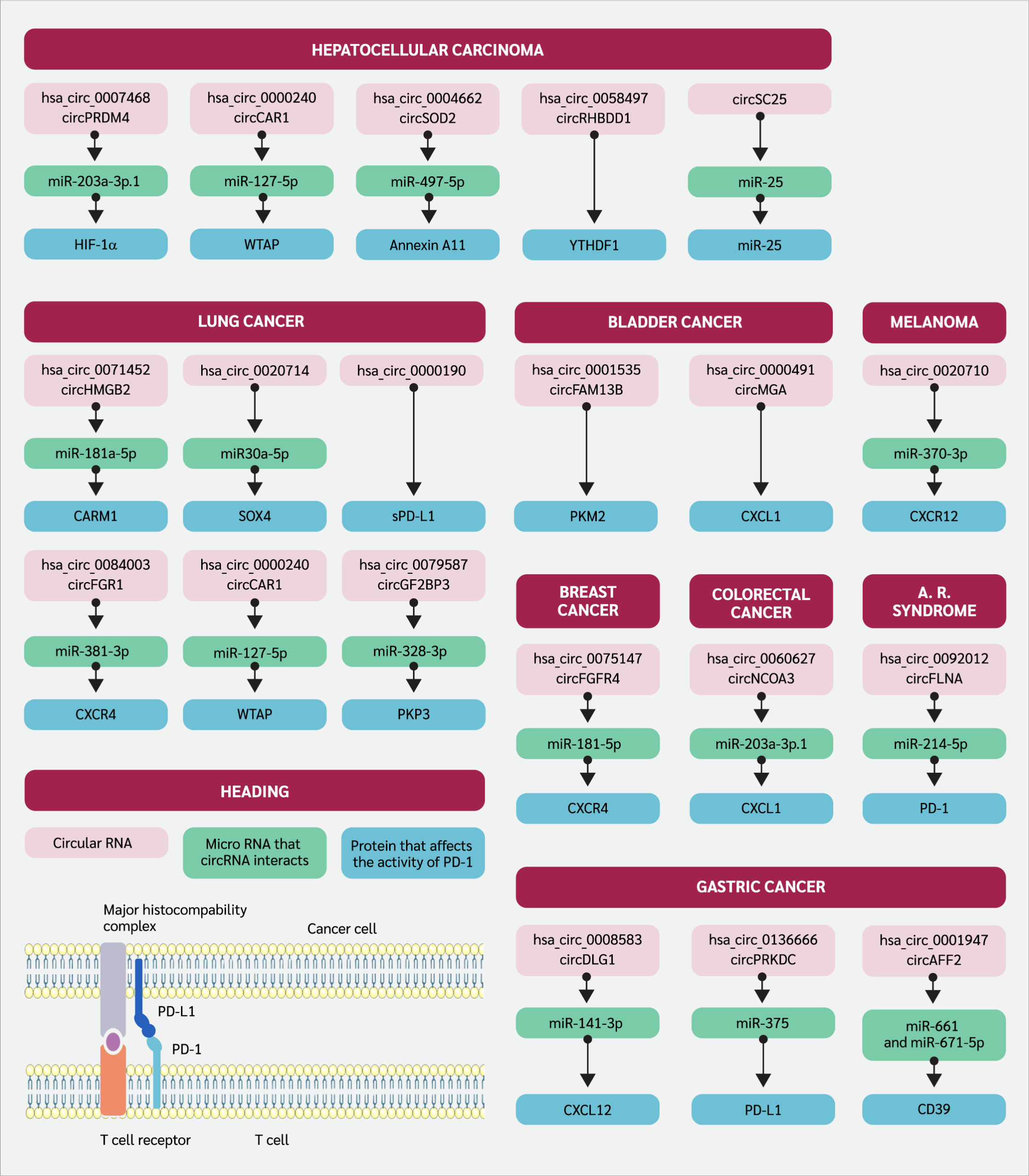
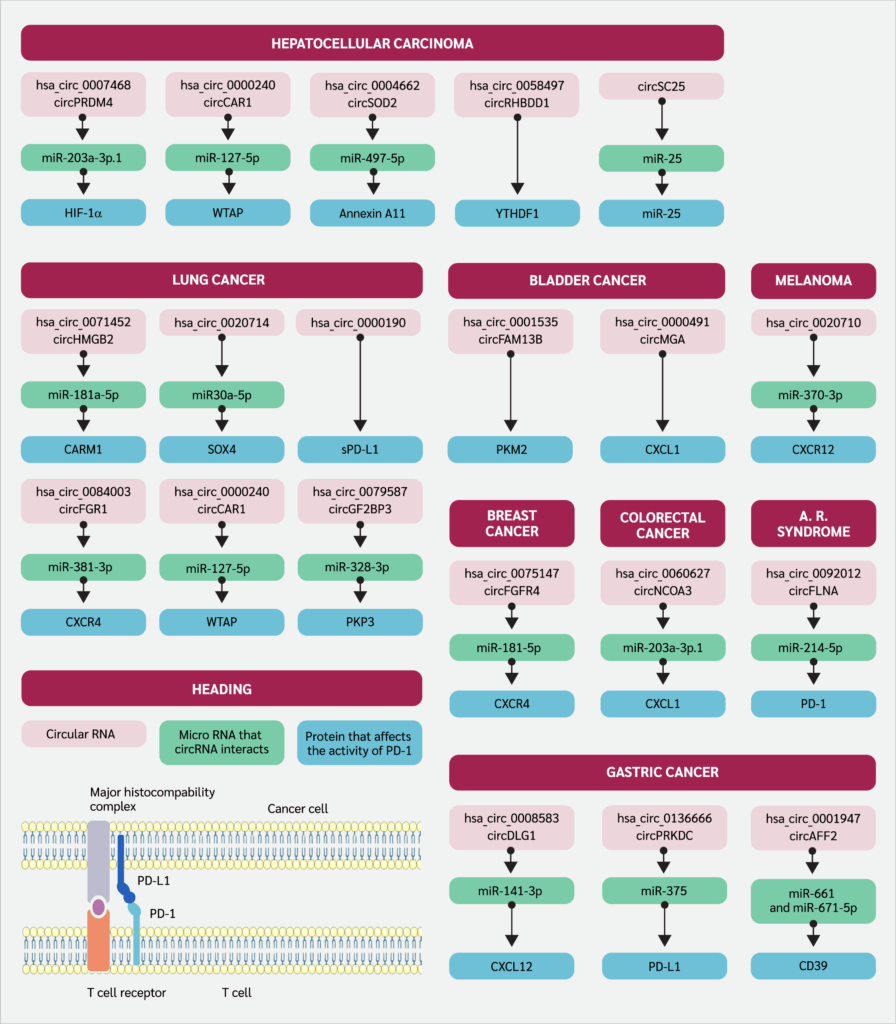
In this review, we aimed to reveal the influence of circRNAs on anti-programmed cell death 1 (anti-PD-1) therapy and their underlying pathways, which give us insights into considering circRNAs either as interrupting or promoting the therapeutic efficacy. After a literature search on databases such as Scopus, Web of Science, and PubMed, all the relevant information was summarized for each different malignancy, separated by the subheadings. Critically, the mechanisms discussed, and data compiled integrate findings from both in vitro (cell lines) and in vivo (animal and clinical) studies, providing a holistic view of the circRNA-PD-1 axis. The identifier of the circRNAs and their downstream pathways were indicated in Figure 1. The affected protein expression levels and the relationship with anti-PD-1 therapeutic efficacy were summarized in Table 1
Circular RNAs and Programmed Cell Death 1 (PD-1) Axis in Multiple Malignancies
Colorectal Cancer
In a study that investigated colorectal cancer (CRC) patients who underwent PD-1 immune checkpoint blockade therapy, it was determined that hsa_circ_0060627, also known as circNCOA3, was associated with an increase in metastatic ability and tumor mass. Furthermore, levels of circNCOA3 were found to be correlated with low overall survival and progression-free survival. Also in this study, results were confirmed to be from the activity of circNCOA3 as miR-203a-3p.1 sponge, which eventually inhibits the C-X-C motif chemokine ligand 1 (CXCL1) mRNA transcription. The downstream effect of these mechanisms can be summarized as the accumulation of MDSCs, which usually prevent the effector functions of immune cells in the tumor microenvironment. Also, it was proposed to be a means suitable for possible therapeutic intervention as augmenting PD-1 responsiveness (20).
Gastric Cancer
There is just one study that explores the axis in which gastric cancer (GC) and circRNA are investigated together. As reported by Chen et al. (21), circDLG1 (hsa_circ_0008583) causes the attraction of MDSCs to the tumor site through the C-X-C motif chemokine ligand 12 (CXCL12), which facilitates this migration when circDLG1 performs miR-141-3p sponge activity. The overall effects of this migration can be summarized as diminished cancer cell detection and killing, and insensitivity to anti-PD-1 treatment. Further research revealed that circ_0001947, encapsulated within small extracellular vesicles (sEVs) derived from GC cells, is markedly upregulated in GC and is associated with unfavorable clinical outcomes. Functionally, elevated circ_0001947 levels enhance GC cell proliferation, migration, and invasion. Mechanistically, circ_0001947 functions as a molecular sponge for miR-661 and miR-671-5p, resulting in increased CD39 expression, which in turn promotes CD8+ T cell exhaustion and contributes to immune evasion. In contrast, inhibition of circ_0001947 mitigates CD8+ T cell exhaustion and improves the efficacy of anti-PD-1 therapy (31). Additionally, hsa_circ_0136666 has been shown to act by sponging miR-375-3p to competitively upregulate protein kinase, DNA-activated, catalytic subunit (PRKDC) expression, which regulates immune checkpoint proteins and promotes PD-L1 phosphorylation to prevent its degradation, thereby driving PD-L1 aggregation and suppressing immune function (33).
Pancreatic Cancer
According to a study by Zhao et al. (34) covering pancreatic adenocarcinoma, the researchers specifically examined the involvement of circ-UBAP2 within the circRNA network. Through their analysis, circ-UBAP2 was identified as a key regulator influencing the expression of pivotal genes, including C-X-C motif chemokine receptor 4 (CXCR4) and zinc finger E-box binding homeobox 1 (ZEB1), in tumor tissues. Furthermore, it was observed that circ-UBAP2 interacts with hsa-miR-494 to modulate the expression levels of these genes. This interaction underlines the potential role of circ-UBAP2 in orchestrating immune modulation within the tumor site, potentially impacting immune cell infiltration and function through increased PD-1 levels.
Hepatocellular Carcinoma
In hepatocellular carcinoma (HCC), according to a study done by Chen et al. (26), hsa_circ_0007468, also known as circPRDM4, was evaluated in hypoxic conditions. It was found that circPRDM4 was associated with increased PD-L1 levels through the HIF-1α pathway, which is correlated with the positive response to anti-PD-1 therapy with good prognostic markers; however, downstream activation of PD-L1 was reported as diminishing anti-tumor response to the cancerous cells, resulting in immune evasion. In addition, a microarray study comprising HCC patients and their circRNA levels was assessed by Hu et al. (35), and circCCAR1 (hsa_circ_0000240) was discovered to be abundant in patients’ plasma samples, especially located in exosomal content. CircCCAR1 was associated with the regulation of PD-1 through post-transcriptional events, as high circCCAR1 levels were correlated with the diminished proteolysis of PD-1 and ubiquitination, which all affect the resistance to inhibition of PD-1. By using BALB/c nude mice, Ye et al. (36) established a mice xenograft tumor assay for understanding the role of hsa_circ_0004662 or circSOD2 in HCC, as a result of the study it was confirmed that circSOD2 through sponging the miR-497-5p upregulate the oncogene expression such as Annexin A11 (ANXA11) which is also thought to be involved in cancer hallmarks as favoring tumor survival. This pathway and its axis were also found to be correlated with poor response to anti-PD1 therapy. CircRHBDD1 (hsa_circ_0058497) in HCC throughout the mechanism that upregulates glycolysis, as its elevated levels were identified to be associated with prolonged HCC progression. The underlying mechanism in the context of glycolysis and circRHBDD1 was considered as YTH domain-containing family protein 1 (YTHDF1), as they recognize post-transcriptional RNA modification m6A and upregulate the translational activity of phosphoinositide-3-kinase regulatory subunit 1 (PIK3R1). Also, when considering circRHBDD1 as an indicator of the potency of PD-1 blockade, elevated levels were detected in patients with low response rates to the anti-PD-1 therapy (37). In another study, Lai et al. (38). developed an innovative, multifunctional engineered circRNA for HCC therapy, designed to execute a dual therapeutic function: it acts as a sponge for the oncogenic miR-25 while simultaneously expressing an anti-PD-1 single-chain variable fragment (scFv). This circRNA-based construct successfully inhibited HCC cell proliferation and suppressed angiogenesis in vitro by effectively sequestering miR-25. Furthermore, the expression of the anti-PD-1 scFv significantly bolstered the cytotoxic T-cell response against the cancer cells, indicating enhanced immunotherapeutic action. The combined mechanism proved highly effective in vivo, resulting in a significant reduction in tumor volume and prolonged survival in treated mouse models compared to controls, thereby demonstrating a promising strategy for combined gene- and immuno-therapy in HCC
Bladder Cancer
Via utilizing NOG (NOD/Shi-scid/IL-2Rγnull) mice, which are immunodeficient as they do not have some of the immune cells or even their immune regulatory functions are diminished, the researchers created a humanized (HuNOG) bladder cancer model by injecting human peripheral blood mononuclear cells (PBMCs) and bladder cancer cell line T24. NOG mice are immunodeficient as they do not have some immune cells or their immune regulatory functions are diminished. Researchers created a humanized (HuNOG) bladder cancer model by injecting PBMC and the bladder cancer cell line T24. This model is used to study the interactions between the human immune system and cancer cells. By working with HuNOG, Lv et al. (39) stated that, after two weeks of PD-1 immunotherapy, circFAM13B was found to be as effective in improving therapeutic efficacy in the case of bladder cancer as compared to mice with suppressed circFAM13B expression through reversing the enzymes promoting lactic acid formation (pyruvate kinase muscle isozyme M2 [PKM2]) and eventually increased pH levels can restore effector functions of immune cells in the TME.
Furthermore, hsa_circ_0000591 (circMGA) was found to be correlated with an increased level of chemokine ligand 5 (CCL5), which is responsible for the enrollment of CD8+ cytotoxic T cells to the TME through the mechanism of CCL5 mRNA stabilization with the help of heterogenous nuclear ribonucleoprotein L (HNRNPL) and these results reflected the capability of this mechanism to induce efficient anti-PD-1 therapy (40).
Breast Cancer
With the research done by Wang et al. (41) in triple-negative breast cancer (TNBC) lesions, it was found that the severity of the disease course was correlated with the elevated levels of circFGFR4 (hsa_circ_0075147). The research findings demonstrated that circFGFR4 promotes immune escape in TNBC and is a predictive biomarker for anti-PD-1 immunotherapy responsivity.
Mechanistically, circFGFR4, when overexpressed, competitively sequesters miR-185-5p (acts like a sponge), preventing the suppression of CXCR4 expression and thereby hindering CD8+ cytotoxic T cell infiltration into TNBC lesions in both human and mice. Moreover, circFGFR4 emerges as a potential therapeutic target in TNBC patients, particularly those undergoing anti-PD-1 immunotherapy.
Lung Cancer
The dual effect of circHSP90A (hsa_circ_0033393) was shown as an activator of the signal transducer and activator of transcription 3 (STAT3) pathway and elevated levels of PD-L1 in non-small cell lung cancer (NSCLC). Circ_HSP90A recruits and increases the levels of ubiquitin-specific peptidase 30 (USP30), which is responsible for the downstream activation of heat shock protein 90 A (HSP90A) via ubiquitination and eventually STAT3 activation that is thought to be related to the upregulation in cancer stem cell formation, invasion, and migratory ability. On the other hand, throughout the miR-424-5p sponge effect, circ_HSP90A increases the level of PD-L1 expression, which ultimately causes programmed cell death of CD8+ cytotoxic T cells, characterized by diminished anti-tumor response (42). Another study, revealing the circRNA and NSCLC axis, was done by Tian et al. (43), who stated that circ_001678, which captures miR-326, leads to diminished miR-326 levels. This study has governed the rise in the levels of PD-L1 throughout the ZEB1 protein, causing lung cancer cell proliferation and depletion in apoptosis or cytotoxicity maintained by CD8+ T cells via PD-L1 overexpression. In NSCLC, circHMGB2 (hsa_circ_0071452) showed increased expression in lung tumor tissues, correlating with poor prognosis in both adenocarcinoma and squamous cell carcinoma patients, which are the common subtypes of NSCLC. While its impact on cell proliferation was moderate, circHMGB2 notably influenced the TME by inducing antitumor immune cell exhaustion, which ultimately downregulates the effectiveness of monoclonal antibody therapy targeting PD-1. circHMGB2 facilitated the downregulation of the type 1 interferon response by sponging miR-181a-5p, thereby enhancing coactivator-arginine methyltransferase 1 (CARM1) activity (44). As remarked by a study done by Wu et al. (45), a high level of hsa_circ_0020714 expression was defined in NSCLC tumors as compared to surrounding healthy tissue. By sponging miR-30a-5p, this context results in an increased expression of transcription factor ex-determining region Y-box 4 (SOX4), which is classified as an oncogene and eventually causes impairment in response to anti-PD-1 therapy. Hsa_circ_0000190, another type of circular RNA, has been identified as a potential biomarker for monitoring the progression of NSCLC and assessing the effectiveness of immunotherapy. Research conducted by Luo et al. (46) suggested that elevated levels of hsa_circ_0000190 may contribute to poor prognosis in NSCLC patients by regulating the expression of soluble PD-L1 (sPD-L1), which has a negative influence on anti-PD-L1 antibody therapeutic efficiency and cytotoxic T-cell activation. This study implies a role for hsa_circ_0000190 in promoting tumorigenesis and immune evasion in NSCLC, which suggests an antagonistic approach to defined circRNAs’ levels could potentially enhance the effectiveness of immunotherapy in treating NSCLC. In addition, Zhang et al. (47) delved into the upregulated expression of circFGFR1 within NSCLC tissues, correlating with an adverse clinical profile and poor prognosis in NSCLC patients. Insights from this research underline circFGFR1's role as directly sponging miR-381-3p and downregulating its downstream target, CXCR4. This intricate regulatory network orchestrated by circFGFR1 confers NSCLC progression and resistance to anti-PD-1 immunotherapy. Lastly, in NSCLC, increased expression of circIGF2BP3 was found to be associated with reduced CD8+ T cell infiltration and compromised anti-tumor immunity in mice, mediated by plakophilin 3 (PKP3) and PD-L1. Via revealing the pathways correlated with the circIGF2BP3, N6-adenosine-methyltransferase 70 kDa subunit (METTL3) facilitates the modification of circIGF2BP3, which competitively upregulates PKP3 by sponging miR-328-3p and miR-3173-5p. Another finding that researchers postulated implies that inhibition of circIGF2BP3/PKP3 enhances the effectiveness of anti-PD-1 therapy in NSCLC mouse models, which is thought to be a possible therapeutic strategy (48).
Metastatic Melanoma
By using the bioinformatics analysis of RNA sequence data taken from different databases, metastatic melanoma (MM) patients who are on the course of anti-PD-1 treatment alone were analyzed to elucidate circRNAs that hold the potential to emphasize prognostic markers related to the monotherapy of PD-1 blockade. Among more than 74,000 distinct circRNAs, five of them were statistically shown to be related to response or survival rates in MM patients throughout the treatment regime, either pembrolizumab or nivolumab, as PD-1 blockers (49). RNA sequencing of cutaneous melanoma patients’ formalin-fixed and paraffin-embedded samples with a history of metastasis was done to define possible circRNA candidates that are potential markers of therapeutic efficacy among the patient cohort taking nivolumab as chemotherapy, which is an anti-PD-1 monoclonal antibody. Together with the other circRNA candidates, it was shown that the hsa_CDR1_0001 signifi cantly increased, and it is thought to be associated with response to nivolumab chemotherapy according to statistical analysis, which needs further validation with in vitro or in vivo studies (50). Researchers discovered that circ_0020710, found in higher levels in melanoma samples, correlates with more aggressive cancer behavior and poorer patient prognosis. Formation of this circRNA causes upregulation in CXCL12 expression through miR-370-3p sponging, which ultimately leads to activation of biochemical pathways that are essential for tumor progression and even calls out MDSCs, which promotes an unfavorable environment for efficient response to cancer cells by cytotoxic T cells. Researchers also postulated that combining AMD3100 and anti-PD-1 therapies effectively slows tumor growth, offering a potential treatment approach for melanoma (51).
Other Pathologies
As stated by Zhong et al. (26), elevated levels of circFLNA (or hsa_circ_0092012) were found to be associated with the severity of acute respiratory distress syndrome (ARDS) induced by sepsis as examined via bronchoalveolar lavage of the patients and mice, and also with the lung tissue of mice. Inhibition of circFLNA led to a notable increase in CD4+CD25+FoxP3+ Tregs and suppressed the release of inflammatory cytokines in ARDS mice. miR-214-5p, which circFLNA behaves like a sponge to it, played a crucial role in modulating these effects by targeting PD-1, suggesting its potential as a therapeutic target for ARDS. These findings highlight the intricate regulatory network involving circFLNA, miR-214-5p, and PD-1 in the pathogenesis and treatment of ARDS. In a study comparing PD-1 antagonist-treated A/PR8(H1N1) influenza A-infected mice lungs to control groups, differential expression analysis revealed significant changes in various types of non-coding RNA molecules. Specifically, 22 differentially expressed circRNAs were identified in the lungs, while in the spleens of mice, 24 circRNAs showed differential expression. However, it was not shown which circRNA is responsible for impairment in antiviral immunity or the essential pathways regulating viral infection-associated comorbidities; overall results can express their possible role in PD-1 checkpoint blockade treatment (52).
Conclusion
In summary, circRNAs have emerged as a research interest within cancer immunotherapy research, exhibiting considerable potential and clinical relevance, particularly in the context of PD-1 blockade therapy across diverse cancer types. Our literature review of circRNA biogenesis and functionalities has given novel insights into their molecular mechanisms in the context of PD-1 blockade in cancer. CircRNAs exhibit a widened array of functions, serving as miRNA and protein sponges, translational modulators, and gene expression regulators, collectively modulating the efficacy of PD-1 blockade therapy. Nevertheless, despite notable advancements, the exploration of the circRNA-PD-1 blockade axis remains limited and needs further research to apply circRNA-based interventions to clinical practice effectively. Here are some points that need to be addressed: firstly, while studies have postulated individual pathways, a better understanding of multiple pathways' modulating circRNA’s downstream effects and their synergistic effects is needed to ease our understanding of the circRNA network's molecular interactions. Secondly, particular attention should be given to exosomal circRNAs, as they have distinct biological functions, which hold promise in increasing the efficacy of PD-1 blockade therapy. Thirdly, while most of the techniques primarily rely on in vitro cellular assays, blood-based assays, and animal models, extensive clinical investigations and multicenter trials are necessary to advance our understanding of circRNAs in clinical settings. Utilizing new technologies is also important in elucidating the physiological importance of circRNAs, so that it will be possible to integrate them into diagnostic and therapeutic approaches related to PD-1 blockade therapy across different cancer types. Efforts given by researchers will give rise to new therapeutic approaches in cancer immunotherapy, such as involving circRNA in their experimental setup, which may even lead to a new era in cancer treatment. Further studies are needed to determine the therapeutic potential of circRNAs in specific cancer types.
Ethical Approval
N.A.
Informed Consent
N.A.
Peer-review
Externally peer-reviewed
Author Contributions
Concept – D.K., F.A., S.K.T.; Design – D.K., F.A.; Supervision – D.K., F.A., S.K.T.; Data Collection and/or Processing – D.K., F.A.; Analysis and/or Interpretation – D.K., F.A.; Literature Review – D.K., F.A.; Writer – D.K.; Critical Reviews – D.K., F.A., S.K.T.
Conflict of Interest
The authors declare no conflict of interest.
Financial Disclosure
The authors declared that this study has received no financial support
References
Esfahani K, Roudaia L, Buhlaiga N, Del Rincon SV, Papneja N, Miller WH Jr. A review of cancer immunotherapy: from the past, to the present, to the future. Curr Oncol. 2020;27(Suppl 2):S87-S97. [CrossRef]
Wu X, Meng Y, Liu L, Gong G, Zhang H, Hou Y, et al. Insights into non-peptide small-molecule inhibitors of the PD-1/PD-L1 interaction: Development and perspective. Bioorg Med Chem. 2021;33:116038. [CrossRef]
Lee JB, Ha SJ, Kim HR. Clinical insights into novel immune checkpoint inhibitors. Front Pharmacol. 2021;12:681320. [CrossRef]
Lin X, Lu X, Luo G, Xiang H. Progress in PD-1/PD-L1 pathway inhibitors: From biomacromolecules to small molecules. Eur J Med Chem. 2020;186:111876. [CrossRef]
Alturki NA. Review of the immune checkpoint inhibitors in the context of cancer treatment. J Clin Med. 2023;12(13):4301. [CrossRef]
Li Y, Yang L, Liu Z. Combination of immune checkpoint inhibitors and anti-angiogenic agents: An emerging strategy for cancer therapy. Crit Rev Oncol Hematol. 2025;215:104883. [CrossRef]
Nielsen DL, Juhl CB, Chen IM, Wang Y, Nielsen OH, Santomasso BD. Immune checkpoint inhibitor-related neurotoxicity: Incidence and management. A systematic review and meta-analysis. Cancer Treat Rev. 2025;140:103011. [CrossRef]
Kong X, Ou S, Wei Z, Ye X, Chen S, Shi X, et al. Transforming the "cold" tumors to "hot" tumors: strategies for immune activation. Biochem Pharmacol. 2025;241:117194. [CrossRef]
Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):1-11. [CrossRef]
Zak KM, Grudnik P, Magiera K, Dömling A, Dubin G, Holak TA. Structural biology of the immune checkpoint receptor PD-1 and its ligands PD-L1/PD-L2. Structure. 2017;25(8):1163-74. [CrossRef]
Ai L, Xu A, Xu J. Roles of PD-1/PD-L1 pathway: signaling, cancer, and beyond. Adv Exp Med Biol. 2020;1248:33-59. [CrossRef]
Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10(3):727-42.
Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977;74(12):5463-7. [CrossRef]
Liu J, Zhao F, Chen LL, Su S. Dysregulation of circular RNAs in inflammation and cancers. Fundam Res. 2023;3(5):683-91. [CrossRef]
Liu Q, Li S. Exosomal circRNAs: Novel biomarkers and therapeutic targets for urinary tumors. Cancer Lett. 2024;588:216759. [CrossRef]
Saleem A, Khan MU, Zahid T, Khurram I, Ghani MU, Ullah I, et al. Biological role and regulation of circular RNA as an emerging biomarker and potential therapeutic target for cancer. Mol Biol Rep. 2024;51(1):296. [CrossRef]
Sun X, Zhao X, Xu Y, Yan Y, Han L, Wei M, et al. Potential therapeutic strategy for cancer: Multi-dimensional cross-talk between circRNAs and parental genes. Cancer Lett. 2024;588:216794. [CrossRef]
Ashrafizadeh M, Dai J, Torabian P, Nabavi N, Aref AR, Aljabali AAA, et al. Circular RNAs in EMT-driven metastasis regulation: modulation of cancer cell plasticity, tumorigenesis and therapy resistance. Cell Mol Life Sci. 2024;81(1):214. [CrossRef]
Guan L, Hao Q, Shi F, Gao B, Wang M, Zhou X, et al. Regulation of the tumor immune microenvironment by cancer-derived circular RNAs. Cell Death Dis. 2023;14(2):132. [CrossRef]
Chen DL, Chen N, Sheng H, Zhang DS. Circular RNA circNCOA3 promotes tumor progression and anti-PD-1 resistance in colorectal cancer. Cancer Drug Resist. 2024;7:9. [CrossRef]
Chen DL, Sheng H, Zhang DS, Jin Y, Zhao BT, Chen N, et al. The circular RNA circDLG1 promotes gastric cancer progression and anti-PD-1 resistance through the regulation of CXCL12 by sponging miR-141-3p. Mol Cancer. 2021;20(1):166. [CrossRef]
Panda AC. Circular RNAs act as miRNA sponges. Adv Exp Med Biol. 2018;1087:67-79. [CrossRef]
Duan S, Wang S, Huang T, Wang J, Yuan X. circRNAs: Insight into their role in tumor-associated macrophages. Front Oncol. 2021;11:780744. [CrossRef]
Lv C, Chen J, Wang Y, Lin Y. Immunoregulatory role of exosomal circRNAs in the tumor microenvironment. Front Oncol. 2025;15:1453786. [CrossRef]
Yu S, Su S, Wang P, Li J, Chen C, Xin H, et al. Tumor-associated macrophage-induced circMRCKα encodes a peptide to promote glycolysis and progression in hepatocellular carcinoma. Cancer Lett. 2024;591:216872. [CrossRef]
Chen ZQ, Zuo XL, Cai J, Zhang Y, Han GY, Zhang L, et al. Hypoxia-associated circPRDM4 promotes immune escape via HIF-1α regulation of PD-L1 in hepatocellular carcinoma. Exp Hematol Oncol. 2023;12(1):17. [CrossRef]
Wang L, Liang Y, Zhao C, Ma P, Zeng S, Ju D, et al. Regulatory T cells in homeostasis and disease: molecular mechanisms and therapeutic potential. Signal Transduct Target Ther. 2025;10(1): :345. [CrossRef]
Zhong J, Zhang W, Zhang L, Li J, Kang L, Li X. CircFLNA/miR-214 modulates regulatory T cells by regulating PD-1 in acute lung injury induced by sepsis. Autoimmunity. 2023;56(1):2259131. [CrossRef]
Liu X, Zhang Y, Zhou S, Dain L, Mei L, Zhu G. Circular RNA: An emerging frontier in RNA therapeutic targets, RNA therapeutics, and mRNA vaccines. J Control Release. 2022;348:84-94. [CrossRef]
Xu T, Wang M, Jiang L, Ma L, Wan L, Chen Q, et al. CircRNAs in anticancer drug resistance: recent advances and future potential. Mol Cancer. 2020;19(1):127. [CrossRef]
He AT, Liu J, Li F, Yang BB. Targeting circular RNAs as a therapeutic approach: current strategies and challenges. Signal Transduct Target Ther. 2021;6(1):185. [CrossRef]
Wang B, Liu W, Zhang M, Li Y, Tang H, Wang Y, et al. Circ_0001947 encapsulated by small extracellular vesicles promotes gastric cancer progression and anti-PD-1 resistance by modulating CD8+ T cell exhaustion. J Nanobiotechnology. 2024;22(1):563. [CrossRef]
Miao Z, Li J, Wang Y, Shi M, Gu X, Zhang X, et al. Hsa_circ_0136666 stimulates gastric cancer progression and tumor immune escape by regulating the miR-375/PRKDC Axis and PD-L1 phosphorylation. Mol Cancer. 2023;22(1):205. [CrossRef]
Zhao R, Ni J, Lu S, Jiang S, You L, Liu H, et al. CircUBAP2-mediated competing endogenous RNA network modulates tumorigenesis in pancreatic adenocarcinoma. Aging (Albany NY). 2019;11(19):8484-501. [CrossRef]
Hu Z, Chen G, Zhao Y, Gao H, Li L, Yin Y, et al. Exosome-derived circCCAR1 promotes CD8+T-cell dysfunction and anti-PD1 resistance in hepatocellular carcinoma. Mol Cancer. 2023;22(1):55. [CrossRef]
Ye R, Lu X, Liu J, Duan Q, Xiao J, Duan X, et al. CircSOD2 contributes to tumor progression, immune evasion and anti-PD-1 resistance in hepatocellular carcinoma by targeting miR-497-5p/ANXA11 axis. Biochem Genet. 2023;61(2):597-614. [CrossRef]
Cai J, Chen Z, Zhang Y, Wang J, Zhang Z, Wu J, et al. CircRHBDD1 augments metabolic rewiring and restricts immunotherapy efficacy via m⁶A modification in hepatocellular carcinoma. Mol Ther Oncolytics. 2022;24:755-71. [CrossRef]
Lai Y, Wang F, Cai G, Li Y, Weng J, Cai F, et al. Utilization of artificial circular RNAs as miRNA sponges and anti-PD-1 scFv expression platforms to suppress hepatocellular carcinoma progression. Front Immunol. 2025;16:1609165. [CrossRef]
Lv J, Li K, Yu H, Han J, Zhuang J, Yu R, et al. HNRNPL induced circFAM13B increased bladder cancer immunotherapy sensitivity via inhibiting glycolysis through IGF2BP1/PKM2 pathway. J Exp Clin Cancer Res. 2023;42(1):41. [CrossRef]
Sun J, Zhang H, Wei W, Xiao X, Huang C, Wang L, et al. Regulation of CD8+ T cells infiltration and immunotherapy by circMGA/HNRNPL complex in bladder cancer. Oncogene. 2023;42(15):1247-62. [CrossRef]
Wang F, Lu Q, Yu H, Zhang XM. The Circular RNA circFGFR4 facilitates resistance to anti-PD-1 of triple-negative breast cancer by targeting the miR-185-5p/CXCR4 axis. Cancer Manag Res. 2023;15:825-35. [CrossRef]
Lei J, Zhu J, Hui B, Jia C, Yan X, Jiang T, et al. Circ-HSP90A expedites cell growth, stemness, and immune evasion in non-small cell lung cancer by regulating STAT3 signaling and PD-1/PD-L1 checkpoint. Cancer Immunol Immunother. 2023;72(1):101-24. [CrossRef]
Tian Q, Wu T, Zhang X, Xu K, Yin X, Wang X, et al. Immunomodulatory functions of the circ_001678/miRNA-326/ZEB1 axis in non-small cell lung cancer via the regulation of PD-1/PD-L1 pathway. Hum Mol Genet. 2022;31(23):4094-106. [CrossRef]
Zhang LX, Gao J, Long X, Zhang PF, Yang X, Zhu SQ, et al. The circular RNA circHMGB2 drives immunosuppression and anti-PD-1 resistance in lung adenocarcinomas and squamous cell carcinomas via the miR-181a-5p/CARM1 axis. Mol Cancer. 2022;21(1):110. [CrossRef]
Wu J, Zhu MX, Li KS, Peng L, Zhang PF. Circular RNA drives resistance to anti-PD-1 immunotherapy by regulating the miR-30a-5p/SOX4 axis in non-small cell lung cancer. Cancer Drug Resist. 2022;5(2):261-70. [CrossRef]
Luo YH, Yang YP, Chien CS, Yarmishyn AA, Adekunle Ishola A, Chien Y, et al. Circular RNA hsa_circ_0000190 facilitates the tumorigenesis and immune evasion by upregulating the expression of soluble PD-L1 in non-small-cell lung cancer. Int J Mol Sci. 2021;23(1):64. [CrossRef]
Zhang PF, Pei X, Li KS, Jin LN, Wang F, Wu J, et al. Circular RNA circFGFR1 promotes progression and anti-PD-1 resistance by sponging miR-381-3p in non-small cell lung cancer cells. Mol Cancer. 2019;18(1):179. Erratum in: Mol Cancer. 2020;19(1):21. [CrossRef]
Liu Z, Wang T, She Y, Wu K, Gu S, Li L, et al. N6-methyladenosine-modified circIGF2BP3 inhibits CD8+ T-cell responses to facilitate tumor immune evasion by promoting the deubiquitination of PD-L1 in non-small cell lung cancer. Mol Cancer. 2021;20(1):105. [CrossRef]
Oliver J, Onieva JL, Garrido-Barros M, Berciano-Guerrero MÁ, Sánchez-Muñoz A, José Lozano M, et al. Association of circular RNA and long non-coding RNA dysregulation with the clinical response to immune checkpoint blockade in cutaneous metastatic melanoma. Biomedicines. 2022;10(10):2419. [CrossRef]
Zhou JG, Liang R, Wang HT, Jin SH, Hu W, Frey B, et al. Identification and characterization of circular RNAs as novel putative biomarkers to predict anti-PD-1 monotherapy response in metastatic melanoma patients - Knowledge from two independent international studies. Neoplasia. 2023;37:100877. [CrossRef]
Wei CY, Zhu MX, Lu NH, Liu JQ, Yang YW, Zhang Y, et al. Circular RNA circ_0020710 drives tumor progression and immune evasion by regulating the miR-370-3p/CXCL12 axis in melanoma. Mol Cancer. 2020;19(1):84. [CrossRef]
Ou H, Chen K, Chen L, Wu H. Bioinformatic analysis of PD-1 checkpoint blockade response in influenza infection. BMC Genom Data. 2022;23(1):65. [CrossRef]
VOLUME
,
ISSUE
Correspondence
Received
Accepted
Published
Suggested Citation
DOI
License