Dose-Dependent Effects of Maternal Vitamin D₃ on Offspring IL-6 and IL-1β in a Rat Model
Abstract
Objective:
Vitamin D₃ is increasingly recognized for its role in immune regulation, particularly in modulating proinflammatory cytokines during early development. This study aimed to investigate the dose dependent effects of maternal vitamin D₃ supplementation during pregnancy and lactation on offspring inflammatory cytokine levels, focusing on interleukin-1 beta (IL-1β) and interleukin-6 (IL-6).
Materials and Methods:
Twelve pregnant Sprague-Dawley rats were randomly assigned to four groups: one control and three treatment groups receiving vitamin D₃ at 62, 415 and 663 IU/kg body weight/day, respectively. Supplementation was administered orally from gestation day 1 to postnatal day 23. Offspring were subjected to an acute inflammatory challenge via lipopolysaccharide injection, after which induration size was measured. Serum levels of vitamin D₃, IL-1β and IL-6 in six offspring per group were quantified using enzyme-linked immunosorbent assay (ELISA). Data were analyzed by one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) post hoc test.
Results:
No significant difference was observed in post-injection induration size between groups (p>0.05). However, the group receiving 663 IU/kg/day vitamin D₃ exhibited significantly higher serum vitamin D₃ levels (21.15 ± 15.8 ng/mL) compared with controls (3.56 ± 3.20 ng/mL, p=0.023), along with significantly lower IL-1β level (33.5 ± 25.44 pg/mL, p<0.001). IL-6 levels showed a similar decreasing trend. Serum vitamin D₃ was moderately and inversely correlated with IL-1β (r= –0.43, p=0.042).
Conclusion:
Maternal vitamin D₃ supplementation during gestation and lactation elevated serum vitamin D₃ and suppressed IL-1β and IL-6 levels in offspring, suggesting a dose-dependent immunomodulatory effect. These findings highlight the potential of maternal vitamin D₃ status to influence inflammatory responses during early life.
Keywords:
Inflammation IL-1β, and, IL-6 maternal, supplementation vitamin, D₃Highlights
Circular RNAs (circRNAs) have emerged as critical regulators of immune evasion in cancer by modulating programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) signaling pathways.
Recent studies demonstrate that specific circRNAs can directly or indirectly influence PD-1 expression, affecting T cell exhaustion and immune checkpoint blockade efficacy.
Circular RNA-mediated competitive endogenous RNA (ceRNA) networks represent promising therapeutic targets to enhance the response to PD-1 inhibitors.
Major unanswered questions include the precise mechanisms by which circRNAs modulate immune cell phenotypes within the tumor microenvironment and their clinical utility as predictive biomarkers.
Future perspectives suggest integrating circRNA profiles into immunotherapy decision algorithms and exploring circRNA-based therapeutics in combination with immune checkpoint blockade.
Introduction
Vitamin D₃ deficiency is a significant health concern during pregnancy and lactation due to its potential long-term effects on both maternal and neonatal health (1,2). Vitamin D₃ supports calcium homeostasis, immune development, and inflammation regulation (3). Low maternal vitamin D₃ levels are associated with an increased risk of pregnancy complications, including preeclampsia and gestational diabetes, and studies have shown higher rates of neonatal asphyxia and immune dysregulation in infants born to vitamin D₃-deficient mothers (4). Boskabadi et al. (5) reported a higher prevalence of respiratory complications in premature infants of vitamin D₃-deficient mothers, highlighting the link between maternal vitamin D₃ status and neonatal outcomes (5). Furthermore, maternal deficiency reduces neonatal vitamin D₃ levels, increasing susceptibility to immune dysregulation and excessive inflammation (6,7).
During pregnancy and lactation, vitamin D₃ is transferred to the fetus and infant through the placenta and breast milk. It contributes to modulating immune responses by balancing cytokine production, thereby reducing the risk of allergies, chronic inflammation, and other immune-mediated conditions in early life (8). Studies have demonstrated that adequate maternal vitamin D₃ levels during pregnancy decrease the risk of chronic inflammation in infants and promote immune balance, thereby lowering the likelihood of allergic diseases and asthma (9,10). Notably, vitamin D₃ supplementation during pregnancy and lactation has been shown to promote immune homeostasis and reduce inflammation in offspring (9,11).
Vitamin D₃ deficiency has been associated with increased levels of pro-inflammatory cytokines, particularly interleukin- 6 (IL-6) and interleukin-1 beta (IL-1β), which are implicated in the development of inflammatory disorders in children (12). Although vitamin D₃ is widely recognized for its role in immune modulation, limited research has explored its dose-dependent impact during both pregnancy and lactation on offspring cytokine responses to acute inflammatory stimuli (13). To address this gap, the present study investigated the immunomodulatory effects of varying doses of maternal vitamin D₃ supplementation administered throughout gestation and lactation, with a focus on IL-6 and IL-1β expression in the offspring. A rat model is used to simulate maternal and offspring interactions and to support the development of early prevention strategies for inflammatory diseases.
No preclinical study has simultaneously implemented prenatal (gestational & lactational) vitamin D₃ supplementation, followed by acute lipopolysaccharide (LPS)-induced inflammation in offspring, with serum measurement of IL-6 and IL-1β at the 3-hour post-injection timepoint. Prior studies have focused on single markers (e.g., tumor necrosis factor alpha [TNF-α] without LPS challenge), organ-specific inflammation, or descriptive immune modulation without acute challenge. Our design fills this critical knowledge gap by targeting downstream cytokine responses in a time-sensitive, systemic inflammatory context.
Materials and Methods
Experimental Animals
Female founder (F0) Rattus norvegicus (Sprague-Dawley strain) rats were used as experimental animals. The animals were housed in a controlled environment at a temperature of 22 ± 2°C, with a relative humidity of 40–70% and a 12/12-hour light/dark cycle. They had unrestricted access to water and were provided with standard feed (30 grams/day) ad libitum. The study was conducted at the Experimental Animal Laboratory, Faculty of Medicine, Universitas Baiturrahmah, West Sumatra, Indonesia, in accordance with the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines. All experimental procedures were approved by the Research Ethics Committee of the Faculty of Medicine, Universitas Baiturrahmah, West Sumatra, Indonesia (Approval No. 040/ETIK-FKUNBRAH/03/07/2024).
Study Design
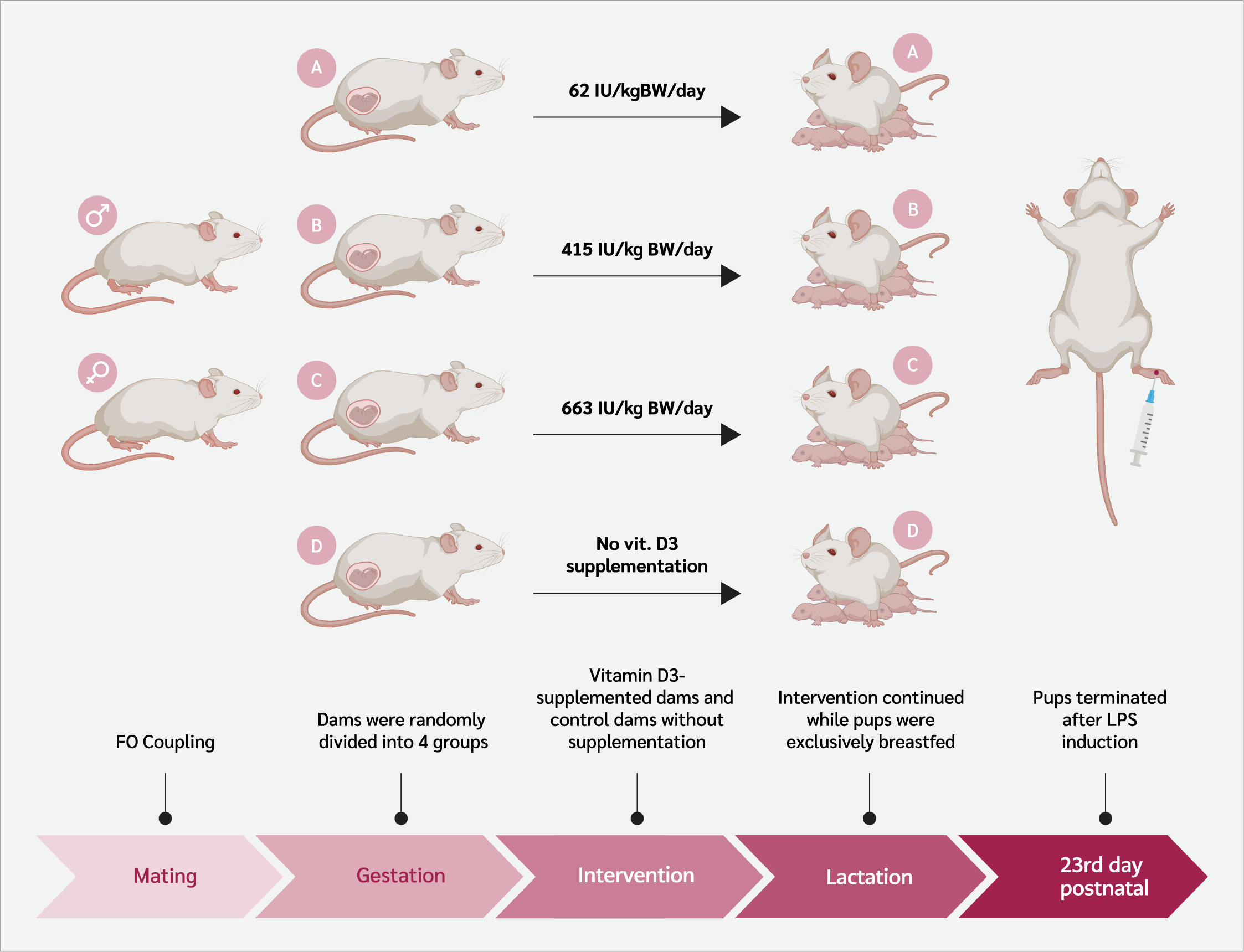
This experimental in vivo study included 12 female Sprague Dawley rats (150–200 g, 8–12 weeks old). The schematic flow of the study is shown in Figure 1. All founder rats underwent a 14-day acclimatization phase before any intervention. After a 14-day acclimatization, founder rats (F0) were paired with males (1 male: 2 females per cage). Pregnancy was confirmed by the presence of a vaginal plug, marking gestational day 1, after which pregnant rats were housed individually (14).
Pregnant rats were randomly assigned to four groups. Three groups received standard feed diet combined with vitamin D₃ (Cat. No. 020734; SUPRA FERBINDO FARMA®, Jakarta, Indonesia) supplementation administered orally via gavage, with each group assigned to a specific supplementation dose (Supplement 1). Group A received 62 IU/kg body weight (BW)/day of vitamin D₃, Group B received 415 IU/kg BW/day of vitamin D₃, and Group C received 663 IU/kg BW/day of vitamin D₃. The vitamin D supplementation was provided separately from the standard diet to ensure accurate dosage administration. Group D served as the control and received no vitamin D₃ supplementation.
The supplementation doses were adjusted to experimental requirements using the Animal Equivalent Dose (AED) conversion formula based on the Km ratio. The human-to-rat dose conversion formula was:
AED (mg/kg) = Human dose (mg/kg) × Km ratio,
Where the Km value for rats is 6.2, and then the results are converted to IU/kg BW. Accordingly, 62 IU/kg BW/day in rats corresponds to 600 IU/day in humans, 415 IU/kg BW/day corresponds to 4000 IU/day, and 663 IU/kg BW/day corresponds to 6400 IU/day. These adjustments ensured the administered doses were physiologically relevant across species.
Rats received 30 grams of feed daily ad libitum and vitamin D₃ administered by oral gavage from gestational day 1 to postnatal day 23. Daily feed intake was measured, including spilled pellets collected from the sawdust. Before weaning on postnatal day 23, six offspring (according to Federer’s formula) with similar body weights per group were selected randomly, regardless of sex, for analysis of serum vitamin D₃ levels, acute inflammation response, edema, and serum IL-1β and IL-6 levels.
Inflammation Induction
On postnatal day 23, inflammation was induced in each offspring with a 100 µg/100 µL LPS (Cat. No. L2630-10MG; Sigma-Aldrich®, Darmstadt, Germany) injection into the paw (0.1 mL). The inflammatory response was assessed by measuring induration 3 hours post-injection, as described by Vajja (15), who found that pro-inflammatory cytokines, such as IL-6 and IL-1β, increased at 3 hours post-LPS injection.
Sample Collection and Biochemical Analysis
Blood samples were collected from the left ventricle. Approximately 2 mL of blood was collected directly from the heart following euthanasia, which was performed using intraperitoneal administration of ketamine at a dose of 100 mg/kg BW. The collected blood was centrifuged at 5000 rpm for 10 minutes to separate the serum. The supernatant was transferred to a new tube and stored at -20°C. Serum vitamin D₃ concentrations were measured using the competitive enzyme-linked immunosorbent assay (ELISA) kit (Cat. No. E-EL-0014; Elabscience®, USA). The detection limit for vitamin D₃ was 0.94 ng/mL, and the intra-assay coefficients were <10%. IL-1β and IL-6 concentrations were assessed using sandwich ELISA kits (Cat. No. E-EL-R0012; Elabscience®, Houston, TX, USA for IL-1β and Cat. No. E-EL-R0015; Elabscience®, Houston, TX, USA for IL-6). The intra-assay coefficients of variation for IL-1β were <10% with a sensitivity of 18.75 pg/mL, while for IL-6, the intra-assay coefficients of variation were <10% with a sensitivity of 7.5 pg/mL.
Statistical Analysis
Data were analyzed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY, USA). The Shapiro-Wilk test was used to assess data normality, and Levene's test was used to evaluate homogeneity of variances. For normally distributed data, two-way analysis of variance (ANOVA) followed by least significant difference (LSD) post hoc testing was performed. For non-normally distributed data, the Kruskal-Wallis test followed by the Mann-Whitney post hoc test was applied. Correlations between variables were examined using Pearson's correlation coefficient. All results were expressed as mean ± standard deviation (SD), and statistical significance was set as p<0.05.
Results
Post-LPS Induction Induration Response
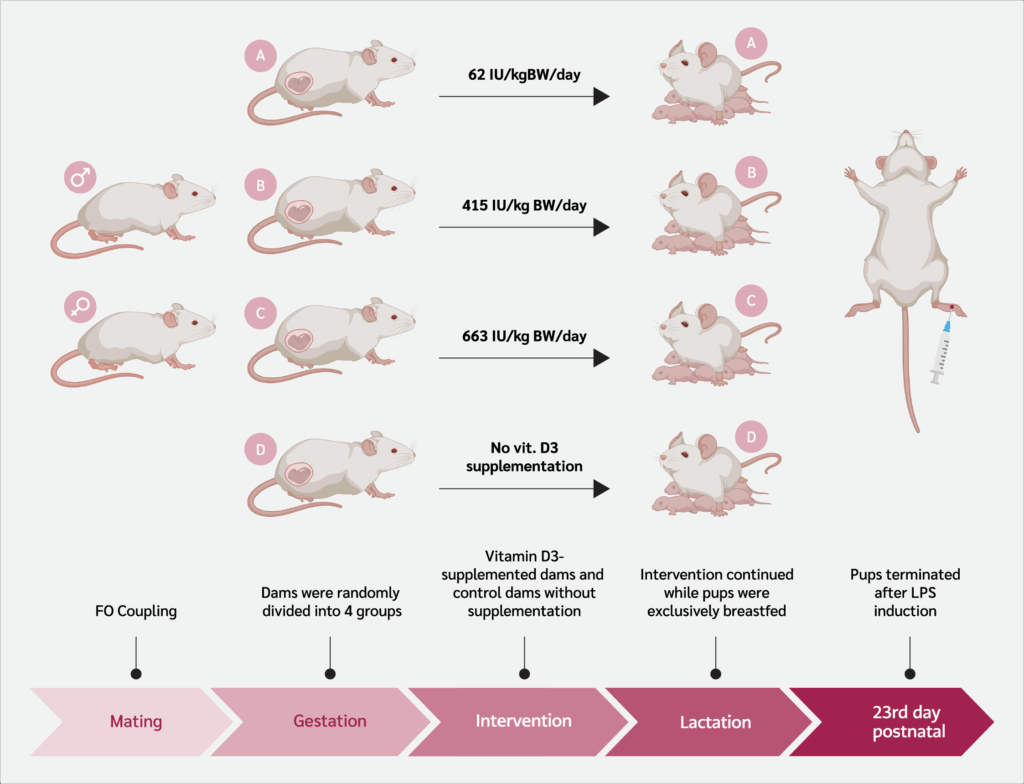
Inflammatory induction was performed by injecting LPS subcutaneously into the plantar surface of the offspring's paw, and induration was measured 3 hours post-injection. The induration measurements (mm) are presented in the graph below (Figure 2). No significant differences in induration diameter were observed among the groups following LPS injection (one-way ANOVA, p>0.05).
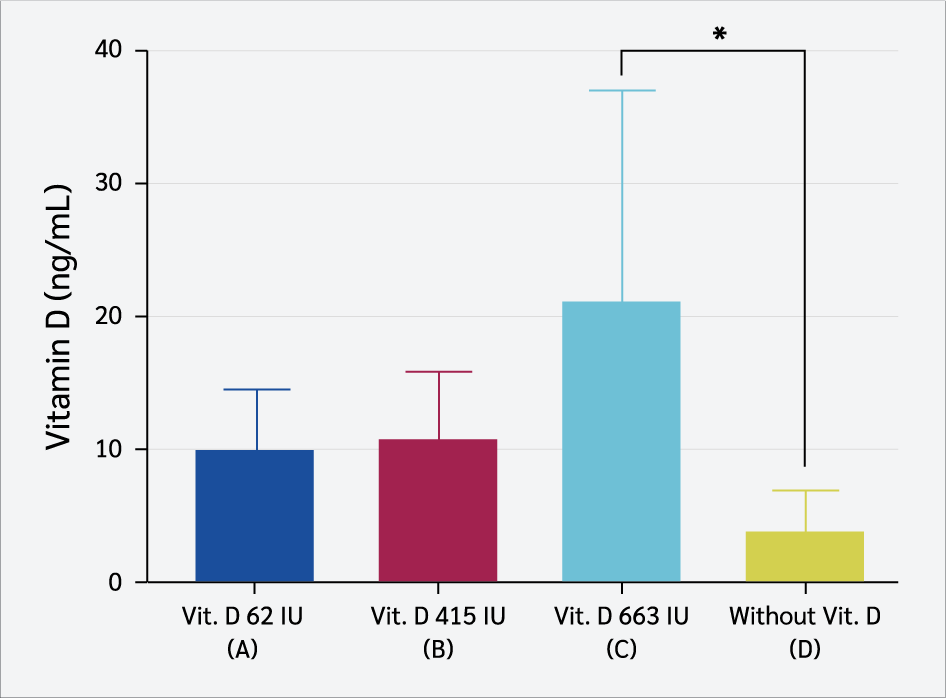
Serum Vitamin D₃ Levels
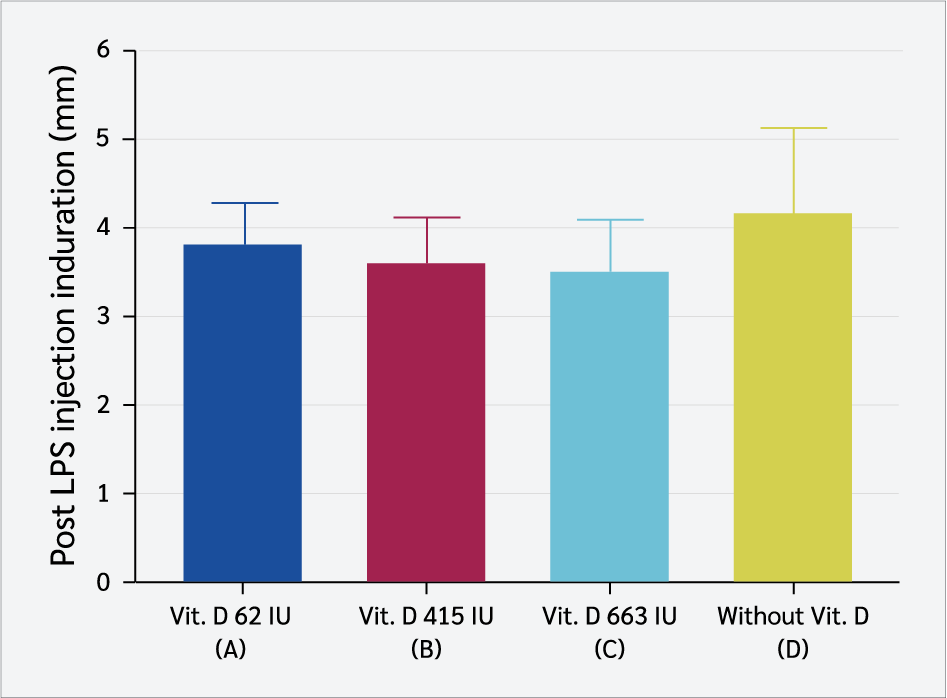
Serum vitamin D₃ levels (ng/mL) in the four groups were assessed on day 23 after LPS induction. This study showed a significant difference in the mean levels (Figure 3) between the group of rats receiving 663 IU/kg BW of vitamin D₃ (21.15 ± 15.8 ng/mL) and the group that did not receive vitamin D₃ (3.56 ± 3.20 ng/mL) (one-way ANOVA, post hoc LSD, p=0.023).
Serum IL-1β level
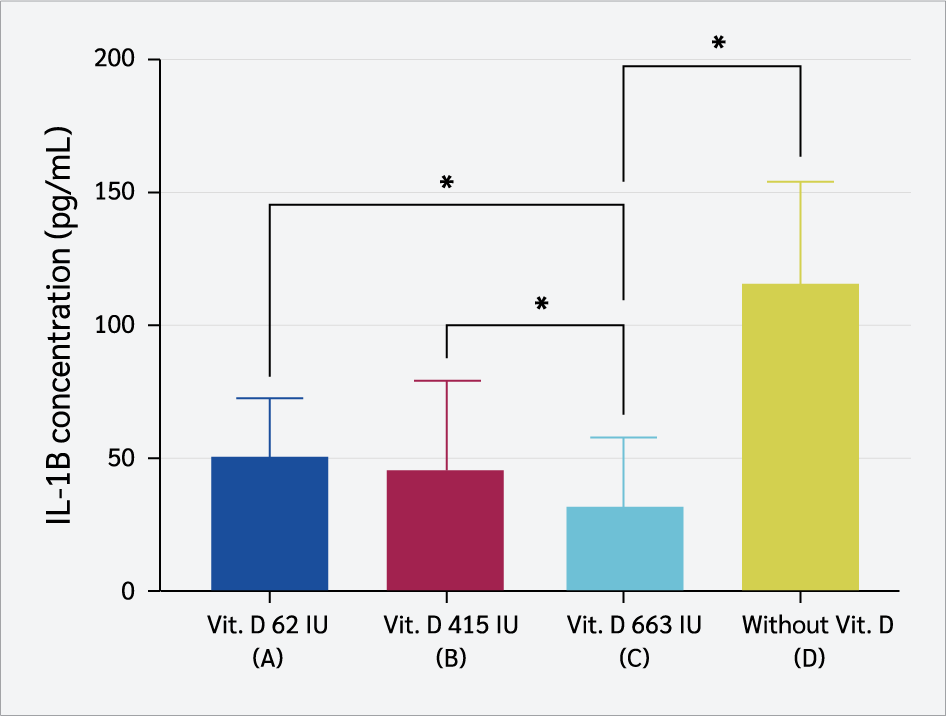
Group C exhibited the lowest serum IL-1β levels, with a mean concentration of 33.5 ± 25.44 pg/mL (Figure 4). Statistical analysis (one-way ANOVA, p<0.001) showed IL-1β levels were significantly higher in low-dose vitamin D₃ groups (62 IU and 415 IU) than in the 663 IU group (LSD post hoc, p=0.03; p=0.02). The non-supplemented group also showed higher IL-1β levels than the 663 IU group (p<0.001), suggesting a modulatory effect of higher vitamin D₃ doses on inflammation.
Serum IL-6 Level
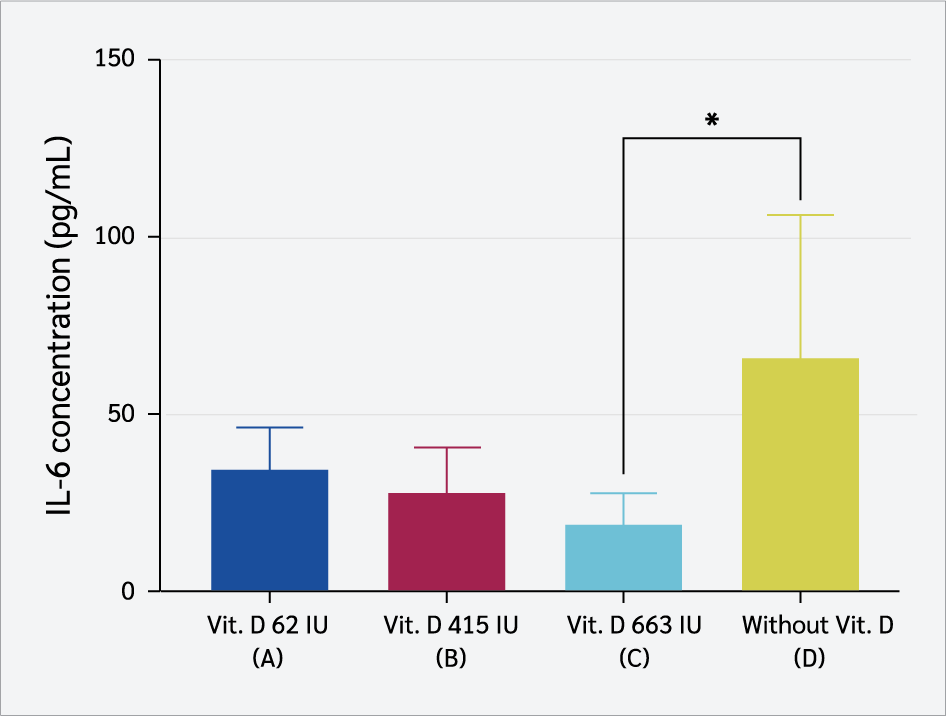
Serum IL-6 levels were lowest in Group C, with a mean of 19.64 ± 9.05 pg/mL (Figure 5). Kruskal-Wallis analysis showed a significant difference among groups (p=0.037), with post hoc LSD revealing lower IL-6 levels in Group C than the non-supplemented group (p=0.023).
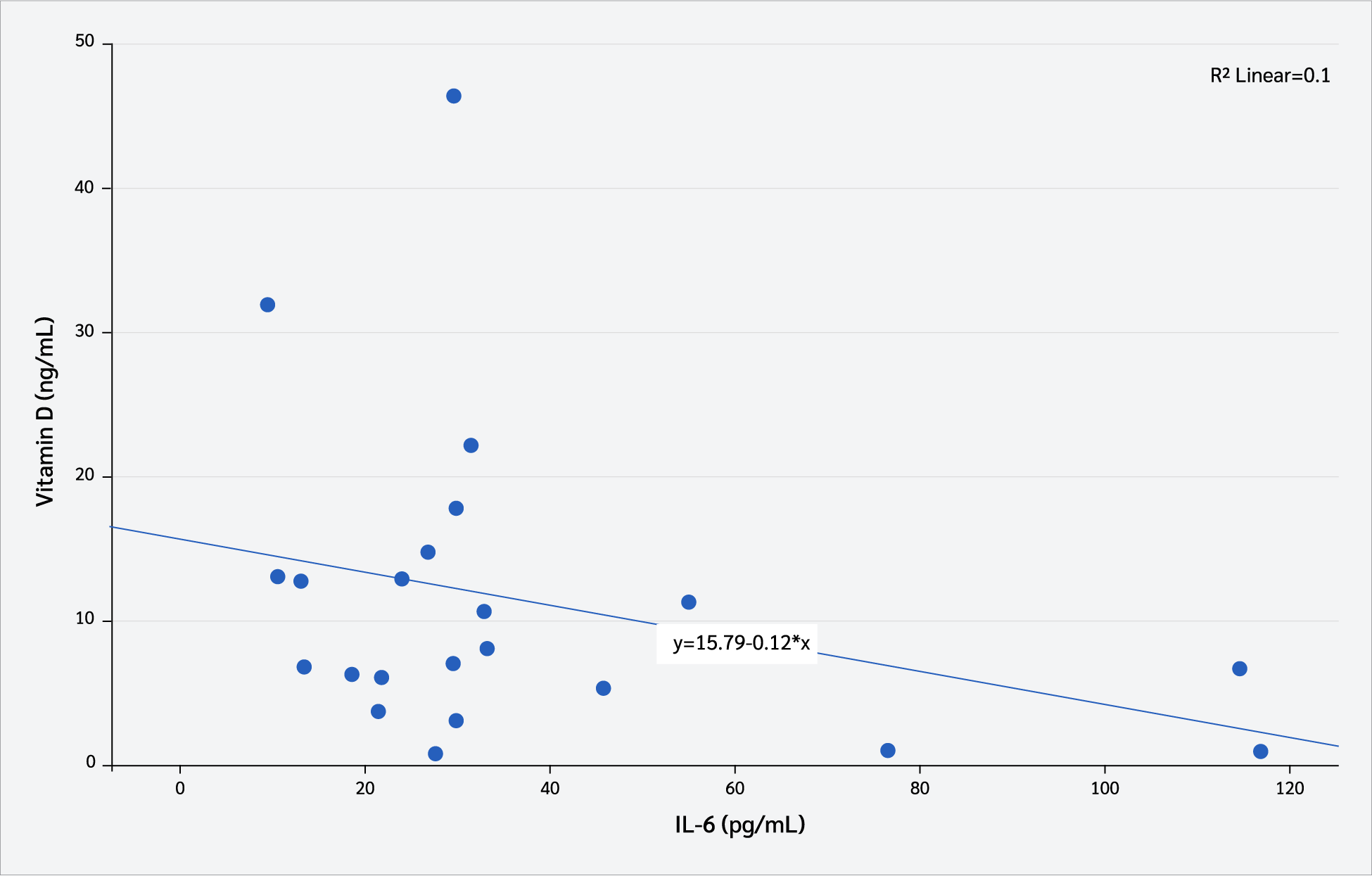
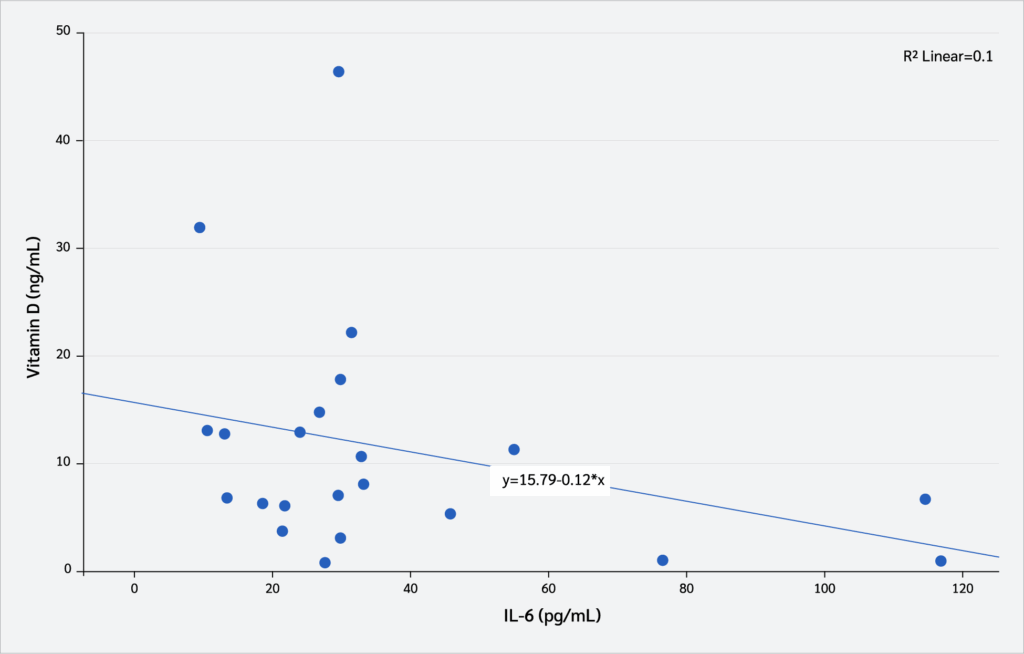
Correlation Between Serum Vitamin D₃ Levels and Interleukin Levels
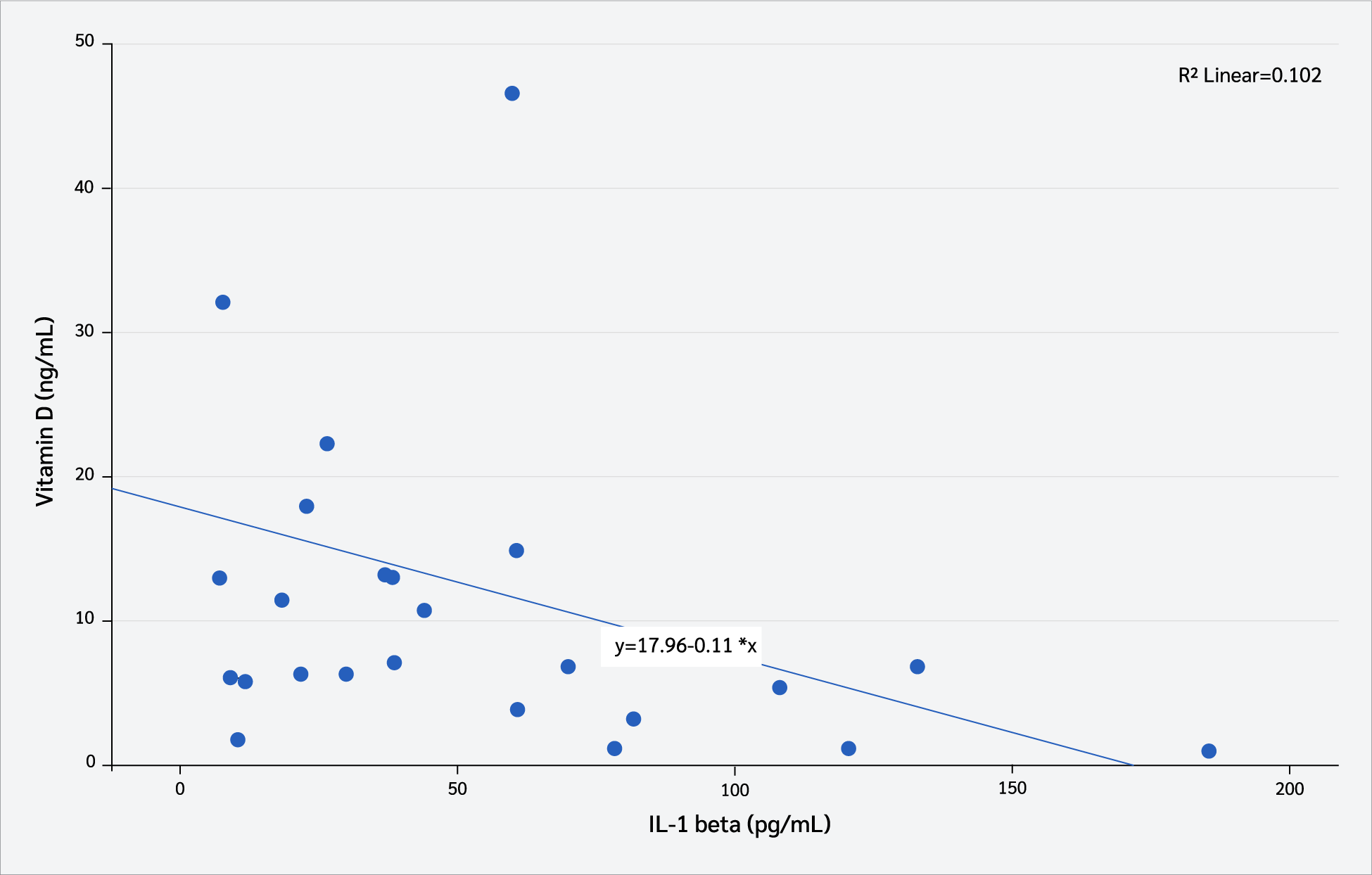
Correlation analyses were conducted to examine the relationship between serum vitamin D₃ levels (pg/mL) and interleukin levels. As shown in Figure 6, serum vitamin D₃ levels exhibited a moderate negative correlation with serum IL-1β concentrations (Pearson’s correlation, r=-0.43, p=0.042). In contrast, no statistically significant correlation was observed between serum vitamin D₃ levels (pg/mL) and serum IL-6 concentrations (Pearson’s correlation, r=-0.031, p=0.153) (Figure 7).
Discussion
The dosage selection in this study was based on previous research and established recommendations (16,17). A dose of 600 IU/day for humans, equivalent to 63 IU/kg BW in rats, was derived from the Institute of Medicine (IOM) guidelines recommending 400–600 IU/day during pregnancy and lactation. However, studies have shown that this level is insufficient to maintain adequate vitamin D3 status. The maximum dose of 6400 IU/day in humans (663 IU/kg BW in rats) was chosen because evidence indicates it meets maternal requirements during breastfeeding without adverse effects (16). An intermediate dose of 4000 IU/day (415 IU/kg BW in rats) was also included, as previous findings demonstrated its effectiveness in meeting vitamin D needs in lactating mothers (17).
This study investigated the effects of maternal vitamin D₃ supplementation on the offspring’s inflammatory response to LPS, assessing induration diameter as a marker of local inflammation. The results showed no significant differences between groups, suggesting that maternal vitamin D₃ supplementation may not directly influence acute local inflammatory responses.
The physiological mechanism underlying LPS-induced induration involves TLR4-dependent activation of innate immune cells and release of pro-inflammatory cytokines—TNF-α, IL-1β, and IL-6—which drive endothelial activation, chemokine production, leukocyte infiltration, and localized swelling/induration (18,19). Although vitamin D₃ is known to downregulate these cytokines and enhance the production of anti-inflammatory cytokines such as IL-10 (20), its modulatory effects appear to be more prominent in chronic or systemic inflammation rather than in acute, localized immune responses (21). We selected IL-6 and IL-1β as primary pro-inflammatory readouts at 3 hours post-LPS. We did not assay TNF-α because its serum levels are known to peak much earlier (often within 1–2 hours post-stimulation) and rapidly decline, making detection at 3 hours susceptible to false negatives. In contrast, IL-6 and IL-1β have more sustained serum profiles and reliably reflect downstream inflammatory amplification (15,22).
Vitamin D₃ primarily modulates systemic inflammation, with studies linking high doses to reduced serum IL-1β and IL-6, but not localized inflammation (23). Additionally, the neonatal immune system is more tolerogenic. A study by Hughes and Norton (24) highlighted that neonatal immune responses tend to suppress excessive inflammation as a developmental adaptation, which may further limit induration diameter in offspring after maternal vitamin D₃ supplementation (25).
Maternal D₃ supplementation significantly altered the serum vitamin D₃ levels of offspring. Offspring from dams receiving 663 IU/kg BW exhibited markedly higher vitamin D₃ levels compared to the non-supplemented group (p=0.023), consistent with findings by Chien et al. (26), who reported that maternal intake exceeding 400 IU/day was associated with improved maternal and offspring outcomes. A clear dose-dependent response was observed, as the 663 IU/kg BW group showed substantially higher levels than controls (21.15 ± 15.8 ng/mL vs. 3.56 ± 3.20 ng/mL), whereas the 62 IU/kg BW group did not differ significantly from controls, suggesting that low doses are insufficient. This aligns with Hollis et al. (16), who noted that low-dose maternal supplementation fails to raise vitamin D₃ concentrations in breast milk, thereby increasing the risk of neonatal deficiency (27).
Maternal vitamin D₃ supplementation enhances the vitamin D₃ status of offspring through both placental transfer and breast milk, with higher maternal levels directly increasing neonatal vitamin D₃ concentrations (28,29). Hollis et al. (16) further emphasized the critical role of adequate maternal vitamin D₃ in optimal fetal development, underscoring the importance of supplementation during pregnancy (27). Additionally, the placental expression of vitamin D₃ metabolic enzymes, such as CYP24A1 and CYP27B1, is regulated, facilitating the transfer of vitamin D₃ to the fetus (30).
Postnatally, breast milk is a key source of vitamin D₃ for exclusively breastfed infants, and its vitamin D content is strongly dependent on maternal intake; high-dose maternal supplementation (≈6000–6400 IU/day) increases milk vitamin D₃ and achieves infant vitamin D sufficiency comparable to direct infant supplementation, thereby lowering the risk of neonatal deficiency (16,31). Adequate maternal supplementation increases milk vitamin D₃ levels, thereby reducing the risk of neonatal deficiency (32,33). Conversely, exclusive breastfeeding without sufficient maternal vitamin D₃ may result in infant deficiency (33). Clinical studies further emphasize the importance of maternal supplementation in maintaining adequate vitamin D₃ levels in infants (34).
Maternal vitamin D₃ supplementation significantly attenuated offspring inflammatory responses, reflected by lower serum IL-1β and IL-6 levels following LPS-induced inflammation. The highest dose of supplementation (663 IU/kg) produced the most pronounced effect, yielding the lowest IL-1β concentration and indicating a dose-dependent anti-inflammatory effect (p<0.001). In contrast, offspring from dams receiving lower doses (62 IU and 415 IU) and the non-supplemented displayed markedly higher IL-1β levels. These findings support the notion that higher maternal vitamin D₃ intake more effectively mitigates inflammation and are consistent with a previous report describing the immunomodulatory capacity of vitamin D₃ in downregulating pro-inflammatory cytokines and limiting excessive immune activation (35).
IL-1β is a pivotal mediator of systemic inflammation, produced predominantly by monocytes and macrophages in response to pathogens or endotoxins (36). It amplifies inflammatory signaling by upregulating inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) (23,37), while simultaneously promoting the release of IL-6 and TNF-α, thereby sustaining inflammatory cascades and contributing to chronic inflammatory diseases (38-41). In this study, offspring from dams supplemented with 663 IU/kg vitamin D₃ exhibited the lowest IL-1β levels which were significantly lower than those of the non-supplemented group. These results indicate that maternal vitamin D₃ supplementation at higher doses is capable of suppressing acute inflammatory responses by downregulating IL-1β production. Mechanistically, vitamin D₃ exerts these effects by inhibiting NLRP3 inflammasome activation, reducing caspase-1–mediated IL-1β maturation, and promoting macrophage polarization toward the M2 phenotype, which enhances IL-10 secretion while suppressing pro-inflammatory cytokines (42-45). Furthermore, vitamin D₃ downregulates Toll-like receptor 4 (TLR4) and nuclear factor kappa B (NF-κB) signaling, key pathways in LPS-induced IL-1β expression, and restrains Th17 differentiation, thereby controlling IL-17–driven inflammation (46-48).
In addition to IL-1β, maternal vitamin D₃ supplementation also reduced serum IL-6 concentrations in offspring. The lowest IL-6 level was observed in the 663 IU/kg group, which was significantly different from the non-supplemented group. IL-6 is a pleiotropic cytokine secreted by macrophages, dendritic cells, T lymphocytes, and fibroblasts in response to infection or tissue injury (49). It regulates immune cell activation, antibody production, and acute-phase responses, and persistent elevation is linked to chronic inflammatory conditions such as type 2 diabetes, autoimmune diseases, and atherosclerosis (37,40,41). Lipopolysaccharide, the main component of Gram negative bacterial cell walls, stimulates IL-6 production by binding TLR4 and activating NF-κB signaling, which also drives the release of IL-1β and TNF-α (38-41). By suppressing NF-κB activation and preventing its nuclear translocation, vitamin D₃ downregulates IL-6 expression (39,49). Vitamin D₃ also reprograms macrophages from a pro-inflammatory M1 state to an anti-inflammatory M2 state, reduces IL-6 while increasing IL-10 secretion, and inhibits Th17 cells that normally amplify IL-6 production, while supporting regulatory T cells that maintain immune tolerance (23, 50-52). These findings are in line with research by Gatera et al. (52), which reported that vitamin D₃ reduces LPS-induced IL-6 synthesis by interfering with NF-κB signaling. Collectively, these mechanisms demonstrate the capacity of vitamin D₃ to modulate LPS-triggered inflammation through coordinated suppression of both IL-1β and IL-6.
Correlation analyses further confirmed the anti-inflammatory role of vitamin D₃. A moderate negative correlation was observed between serum vitamin D₃ and IL-1β levels, whereas no significant relationship was detected with IL-6. These findings aligned with earlier studies demonstrating that higher vitamin D₃ status was associated with lower IL-1β levels (54,55), and may indicate that IL-1β is a more sensitive biomarker of immunomodulatory effects of vitamin D₃ in acute inflammation. Additional evidence suggests that vitamin D₃ supplementation suppresses both IL-1β and IL-6 by modulating mitogen-activated protein kinase (MAPK) phosphatase-1, thereby alleviating LPS-induced inflammatory responses (39). Taken together, these results provide compelling evidence that maternal vitamin D₃ supplementation not only reduces pro-inflammatory cytokine production in offspring but also enhances immune regulation, supporting its potential role as a preventive strategy against excessive inflammatory activation in early life (56,57).
Conclusion
Maternal vitamin D₃ supplementation during pregnancy and lactation increased offspring serum vitamin D₃ levels and reduced IL-6 and IL-1β concentrations, demonstrating a dose-dependent role in regulating systemic inflammation. The negative correlation between vitamin D₃ and IL-1β highlights its potential as an immunomodulatory agent, promoting immune homeostasis in the offspring.
Ethical Approval
The study was approved by the Research Ethics Committee of the Faculty of Medicine, Universitas Baiturrahmah, on July 3, 2024, with decision number 040/ETIK-FKUNBRAH/03/07/2024.
Informed Consent
N.A.
Peer-review
Externally peer-reviewed
Author Contributions
Concept – K.M.H., W.S., G.K., A.D., L.Z., A.L.M.; Design – K.M.H., W.S., A.D., R.M., M.S.; Supervision – K.M.H., W.S., A.D., R.M.; Fundings – K.M.H., W.S.; Materials – K.M.H., W.S., G.K., A.D., M.S., R.M.; Data Collection and/or Processing – K.M.H., W.S., GF, A.D., M.S.; Analysis and/or Interpretation – K.M.H., W.S., G.K., A.L.M.; Literature Review – K.M.H., W.S., G.K., L.Z.; Writer – K.M.H., W.S., G.K., A.D., R.M., M.S., L.Z., A.L.M.; Critical Reviews – K.M.H., W.S., G.K., A.D., R.M., M.S., L.Z., A.L.M.
Conflict of Interest
The authors declare no conflict of interest.
Financial Disclosure
This study was supported by the PDP KEMDIKBUDRISTEK funding scheme (Grant No. 0459/E5/PG.02.00/2024).
Scientific Presentation
Part of this study was presented at the 31st FAOBMB International Conference, May 22, 2025, Busan, South Korea.
References
Aghajafari F, Nagulesapillai T, Ronksley PE, Tough S C, O’Beirne M, Rabi D M, et al. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ. 2013;346:f1169 [CrossRef]
Lindqvist PG, Silva AT, Gustafsson SA, Gidlöf S. Maternal vitamin D deficiency and fetal distress/birth asphyxia: a population-based nested case-control study. BMJ Open. 2016 Sep 22;6(9):e009733. [CrossRef]
Milajerdi A, Abbasi F, Mousavi SM, Esmaillzadeh A. Maternal vitamin D status and risk of gestational diabetes mellitus: A systematic review and meta-analysis of prospective cohort studies. Clin Nutr. 2021;40(5):2576-86. [CrossRef]
Butola LK, Vagga A, Ambad R, Kanyal D, Jankar J. A systematic review of association between vitamin D levels and pre-eclampsia in pregnant women—an old problem revisited. Int J Res Pharm Sci. 2020;11(SPL4):2910-20. [CrossRef]
Boskabadi H, Zakerihamidi M, Mehrad-Majd H, Ghoflchi S. Evaluation of vitamin D in the diagnosis of infants with respiratory distress, the clinical value: A systematic review and meta-analysis. Paediatr Respir Rev. 2025;53:44-54. [CrossRef]
Cyprian F, Lefkou E, Varoudi K, Girardi G. Immunomodulatory effects of vitamin D in pregnancy and beyond. Front Immunol. 2019;10:2739. [CrossRef]
Karras SN, Fakhoury H, Muscogiuri G, Grant WB, van den Ouweland JM, Colao AM, et al. Maternal vitamin D levels during pregnancy and neonatal health: evidence to date and clinical implications. Ther Adv Musculoskelet Dis. 2016;8(4):124-35. [CrossRef]
Khudri G, Sukmawati D. Exploring hematopoietic stem cell population in human milk and its benefits for infants: A scoping review. Asian Pac J Reprod. 2024;13(3):107-14. [CrossRef]
Ao T, Kikuta J, Ishii M. The effects of vitamin D on immune system and inflammatory diseases. Biomolecules. 2021;11(11):1624. [CrossRef]
Cashman KD, Ritz C, Kiely M, Odin Collaborators. Improved dietary guidelines for vitamin D: Application of individual participant data (IPD)-Level meta-regression analyses. Nutrients. 2017;9(5):469. [CrossRef]
Rizkia CP. Vitamin D and its role in modulating immune system: a narrative literature review. Open Access Indonesian J Med Rev. 2023;3(1):356-61. [CrossRef]
Goldsmith JR. Vitamin D as an immunomodulator: Risks with deficiencies and benefits of supplementation. Healthcare (Basel). 2015;3(2):219-32. [CrossRef]
Chen WJ, Hou XJ, Yang SF, Yin XH, Ren L. Effect of vitamin D supplementation during pregnancy on the Th1/Th2 cell balance of rat offspring. Pharmazie. 2014;69(5):385-90.
Voipio H-M, Nevalainen T. Improved method for vaginal plug detection in rats. Scand J Lab Anim Sci. 1998;25(1). [CrossRef]
Vajja BN, Juluri S, Kumari M, Kole L, Chakrabarti R, Joshi VD. Lipopolysaccharide-induced paw edema model for detection of cytokine modulating anti-inflammatory agents. Int Immunopharmacol. 2004;4(7):901-9. [CrossRef]
Hollis BW, Wagner CL, Howard CR, Ebeling M, Shary JR, Smith PG, et al. Maternal versus infant vitamin d supplementation during lactation: A randomized controlled trial. Pediatrics. 2015;136(4):625-34. Erratum in: Pediatrics. 2019;144(1):e20191063. [CrossRef]
DePender S, Russell MM, DeJager J, Comstock SS. Impact of maternal vitamin D supplementation during breastfeeding on infant serum vitamin D levels: A narrative review of the recent evidence. Children (Basel). 2022;9(12):1863. [CrossRef]
Buters TP, Hameeteman PW, Jansen IME, van Hindevoort FC, Ten Voorde W, Florencia E, et al. Intradermal lipopolysaccharide challenge as an acute in vivo inflammatory model in healthy volunteers. Br J Clin Pharmacol. 2022;88(2):680-90. [CrossRef]
Patil KR, Mahajan UB, Unger BS, Goyal SN, Belemkar S, Surana SJ, et al. Animal models of inflammation for screening of anti-inflammatory drugs: implications for the discovery and development of phytopharmaceuticals. Int J Mol Sci. 2019;20(18):4367. [CrossRef]
Willis KS, Smith DT, Broughton KS, Larson-Meyer DE. Vitamin D status and biomarkers of inflammation in runners. Open Access J Sports Med. 2012;3:35-42. [CrossRef]
Guan J, Karsy M, Brock AA, Eli IM, Ledyard HK, Hawryluk GWJ, et al. A prospective analysis of hypovitaminosis D and mortality in 400 patients in the neurocritical care setting. J Neurosurg. 2017;127(1):1-7. [CrossRef]
Held F, Hoppe E, Cvijovic M, Jirstrand M, Gabrielsson J. Challenge model of TNFα turnover at varying LPS and drug provocations. J Pharmacokinet Pharmacodyn. 2019;46(3):223-40. [CrossRef]
Rai V, Agrawal DK. Immunomodulation of IL-33 and IL-37 with vitamin D in the neointima of coronary artery: A comparative study between balloon angioplasty and stent in hyperlipidemic microswine. Int J Mol Sci. 2021;22(16):8824. [CrossRef]
Hughes DA, Norton R. Vitamin D and respiratory health. Clin Exp Immunol. 2009;158(1):20-5. [CrossRef]
Castillo EC, Hernandez-Cueto MA, Vega-Lopez MA, Lavalle C, Kouri JB, Ortiz-Navarrete V. Effects of vitamin D supplementation during the induction and progression of osteoarthritis in a rat model. Evid Based Complement Alternat Med. 2012;2012:156563. [CrossRef]
Chien MC, Huang CY, Wang JH, Shih CL, Wu P. Effects of vitamin D in pregnancy on maternal and offspring health-related outcomes: An umbrella review of systematic review and meta-analyses. Nutr Diabetes. 2024;14(1):35. [CrossRef]
Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770-3. [CrossRef]
Al-Rubaye WEL, Al-Saeedy BAT, Al-Sattam ZMJ. Vitamin D deficiency/insufficiency and some of its related factors in a sample of Iraqi pregnant women and their neonates at Al-Elwiya Maternity Teaching Hospital during 2019. Al-Kindy Coll Med J. 2021;17(1):35-40. [CrossRef]
Agarwal N, Tandon P. Incidence of vitamin D levels in cord blood of newborns and correlation with maternal vitamin D. Int J Health Sci (Qassim). 2021;5(S1):687-92. [CrossRef]
Ashley B, Simner C, Manousopoulou A, Jenkinson C, Hey F, Frost JM, et al. Placental uptake and metabolism of 25(OH)vitamin D determine its activity within the fetoplacental unit. Elife. 2022;11:e71094. [CrossRef]
Dawodu A, Salameh KM, Al-Janahi NS, Bener A, Elkum N. The effect of high-dose postpartum maternal vitamin D supplementation alone compared with maternal plus infant vitamin D supplementation in breastfeeding infants in a high-risk population. A randomized controlled trial. Nutrients. 2019;11(7):1632. [CrossRef]
Qin LL, Lu FG, Yang SH, Xu HL, Luo BA. Does maternal vitamin D deficiency increase the risk of preterm birth: A meta-analysis of observational studies. Nutrients. 2016;8(5):301. [CrossRef]
Mallick AK, Yadav RK, Sannalli K, Bewal NM. Maternal hypovitaminosis D presenting as late-onset hypocalcemic seizure in a term neonate: a case report. Egyptian Pediatric Association Gazette. 2023;71(1):15. [CrossRef]
Hutabarat M, Wibowo N, Obermayer-Pietsch B, Huppertz B. Impact of vitamin D and vitamin D receptor on the trophoblast survival capacity in preeclampsia. PLoS One. 2018;13(11):e0206725. [CrossRef]
Hoe E, Nathanielsz J, Toh ZQ, Spry L, Marimla R, Balloch A, et al. Anti-inflammatory effects of vitamin D on human immune cells in the context of bacterial infection. Nutrients. 2016;8(12):806. [CrossRef]
Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;281(1):8-27. [CrossRef]
Rodriguez AJ, Mousa A, Ebeling PR, Scott D, de Courten B. Effects of vitamin D supplementation on inflammatory markers in heart failure: a systematic review and meta-analysis of randomized controlled trials. Sci Rep. 2018;8(1):1169. [CrossRef]
Benedetti G, Miossec P. Interleukin 17 contributes to the chronicity of inflammatory diseases such as rheumatoid arthritis. Eur J Immunol. 2014;44(2):339-47. [CrossRef]
Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188(5):2127-35. [CrossRef]
Pasupuleti P, Suchitra MM, Bitla AR, Sachan A. Attenuation of oxidative stress, interleukin-6, high-sensitivity C-reactive protein, plasminogen activator inhibitor-1, and fibrinogen with oral vitamin D supplementation in patients with T2DM having vitamin D deficiency. J Lab Physicians. 2021;14(2):190-6. [CrossRef]
Mansournia MA, Ostadmohammadi V, Doosti-Irani A, Ghayour-Mobarhan M, Ferns G, Akbari H, et al. The effects of vitamin D supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Horm Metab Res. 2018;50(6):429-40. [CrossRef]
Rao Z, Chen X, Wu J, Xiao M, Zhang J, Wang B, et al. Vitamin D Receptor Inhibits NLRP3 Activation by Impeding Its BRCC3-Mediated Deubiquitination. Front Immunol. 2019;10:2783. [CrossRef]
Cao R, Ma Y, Li S, Shen D, Yang S, Wang X, et al. 1,25(OH)2 D3 alleviates DSS-induced ulcerative colitis via inhibiting NLRP3 inflammasome activation. J Leukoc Biol. 2020;108(1):283-95. [CrossRef]
Lowe DW, Hollis BW, Wagner CL, Bass T, Kaufman DA, Horgan MJ, et al. Vitamin D insufficiency in neonatal hypoxic-ischemic encephalopathy. Pediatr Res. 2017;82(1):55-62. [CrossRef]
Agak GW, Qin M, Nobe J, Kim MH, Krutzik SR, Tristan GR, et al. Propionibacterium acnes Induces an IL-17 response in acne vulgaris that is regulated by vitamin A and vitamin D. J Invest Dermatol. 2014;134(2):366-73. [CrossRef]
Ben-Zvi I, Aranow C, Mackay M, Stanevsky A, Kamen DL, Marinescu LM, et al. The impact of vitamin D on dendritic cell function in patients with systemic lupus erythematosus. PLoS One. 2010;5(2):e9193. [CrossRef]
Jiang LJ, Rong ZH, Zhang HF. The changes of Treg and Th17 cells relate to serum 25(OH)D in patients with initial-onset childhood systemic lupus erythematosus. Front Pediatr. 2023;11:1228112. [CrossRef]
Dankers W, Davelaar N, van Hamburg JP, van de Peppel J, Colin EM, Lubberts E. Human memory Th17 cell populations change into anti-inflammatory cells with regulatory capacity upon exposure to active vitamin D. Front Immunol. 2019;10:1504. [CrossRef]
Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. [CrossRef]
Gunasekar P, Swier VJ, Fleegel JP, Boosani CS, Radwan MM, Agrawal DK. Vitamin D and macrophage polarization in epicardial adipose tissue of atherosclerotic swine. PLoS One. 2018;13(10):e0199411. [CrossRef]
Fabri M, Stenger S, Shin DM, Yuk JM, Liu PT, Realegeno S, et al. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011;3(104):104ra102. [CrossRef]
Chen L, Eapen MS, Zosky GR. Vitamin D both facilitates and attenuates the cellular response to lipopolysaccharide. Sci Rep. 2017;7:45172. [CrossRef]
Gatera VA, Lesmana R, Musfiroh I, Judistiani RTD, Setiabudiawan B, Abdulah R. Vitamin D inhibits lipopolysaccharide (LPS)-induced inflammation in A549 cells by downregulating inflammatory cytokines. Med Sci Monit Basic Res. 2021;27:e931481. [CrossRef]
Li G, Lin L, Wang YL, Yang H. 1,25(OH)2D3 protects trophoblasts against insulin resistance and inflammation via suppressing mTOR signaling. Reprod Sci. 2019;26(2):223-32. [CrossRef]
Sun J, Zhong W, Gu Y, Groome LJ, Wang Y. 1,25(OH)2D3 suppresses COX-2 up-regulation and thromboxane production in placental trophoblast cells in response to hypoxic stimulation. Placenta. 2014;35(2):143-5. [CrossRef]
Ma R, Gu Y, Zhao S, Sun J, Groome LJ, Wang Y. Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am J Physiol Endocrinol Metab. 2012;303(7):E928-35. [CrossRef]
Vestergaard AL, Andersen MK, Olesen RV, Bor P, Larsen A. High-dose vitamin D supplementation significantly affects the placental transcriptome. Nutrients. 2023;15(24):5032. [CrossRef]
VOLUME
,
ISSUE
Correspondence
Received
Accepted
Published
Suggested Citation
DOI
License