Effects of Typeable and Non-typeable Haemophilus influenzae on Human CD4+ T Cell Proliferation and Production of Th1 and Th2 Cytokines
Abstract
Objective:
Haemophilus influenzae is a Gram-negative bacterium that commonly colonizes and infects the respiratory tract. The bacterial infection may modulate host immune responses. However, the T cell responses to H. influenzae remain unclear. This study aimed to investigate the CD4+ T cell responses to live strains of typeable H. influenzae (THi) and non-typeable H. influenzae (NTHi) in vitro.
Materials and Methods:
CD4+ T cells were isolated from healthy individuals and infected with live variants of a single strain of THi (132b+, 132b-, 132b-p5-) and NTHi (A950002, A950002p5-, A850052, d1, d3). The CD4+ T cell responses to H. influenzae were investigated in vitro by evaluating the cell proliferation using a [³H]-thymidine incorporation assay and measuring the levels of T helper-1 (Th1) cytokines (interferon-gamma [IFN-γ], tumor necrosis factor-alpha [TNF-α]) and Th2 cytokines (interleukin-5 [IL-5], interleukin-10 [IL-10]) using a human Th1/Th2 cytokine cytometric bead array (CBA).
Results:
Both NTHi strains and THi are bound to CD4+ T cells to variable degrees, and the presence of the P5 protein in H. influenzae P5+ strains increased the binding to CD4+ T cells significantly compared to P5-deficient strains (132b- vs. 132b-p5- [p= 0.0009], A950002 vs. A950002p5 [p=0.0039], d1 vs. d3 [p=0.0014]). THi (132b-, 132b-p5) and NTHi strains (A850052, d1, d3) caused marked inhibition of CD4+ T cell proliferation (p< 0.0001). NTHi strains (A850052, d1, d3) significantly suppressed IFN-γ, TNF-α, IL-5, and IL-10 production (p<0.0001). THi strains 132b- and 132b-p5- markedly suppressed IFN-γ production (p<0.0001). TNF-α production was significantly inhibited by132b- strain (p=0.0002), and A950002 strain (p=0.01). IL-5 production was reduced by all THi and NTHi strains (p<0.0001), while all THi and some NTHi strains (A850052, d1, d3) decreased IL-10 production (p<0.0001).
Conclusion:
These results suggest that the immunosuppressive effects of certain strains of H. influenzae may represent a mechanism by which the bacterium evades the adaptive immune response, facilitating the establishment of respiratory colonization and persistence of the infection.
Keywords:
Haemophilus, influenzae CD4, T, cell, proliferation Th1, and, Th2, cytokinesIntroduction
Haemophilus influenzae bacterium inhabits the upper respiratory tract and may cause local and systemic diseases (1). Haemophilus influenzae strains are classified into capsulate or typeable H. influenzae (THi) and acapsulate or non-typeable H. influenzae (NTHi) based on the expression of a polysaccharide capsule (1). Typeable H. influenzae strains include six serotypes (a–f), with type b being the most virulent of them all (2). Typeable H. influenzae strains can cause severe diseases, including septicemia and meningitis (3,4). Non-typeable H. influenzae strains are characterized by the absence of a polysaccharide capsule, which distinguishes them from typeable strains (5). These strains may colonize the respiratory tract and are often implicated in exacerbation of chronic obstructive pulmonary disease (COPD). Non-typeable H. influenzae is the most prevalent bacterial cause of recurrent otitis media during childhood (6) and COPD exacerbations in humans (7) and mice (8). Furthermore, NTHi promote biofilm formation and exhibit a strong resistance to the immune system's antimicrobial defenses, which enables them to survive in the COPD lung and cause persistent infections that recur (9).
The initial step in bacterial colonization is attaching to specific receptors on human mucosal epithelial cells. Outer membrane proteins (OMPs) P2 and P5 are involved in the binding of NTHi to mucin, whereas binding to mucosal epithelial cells is mediated by pili (10), high-molecular-weight surface proteins (HMW1 and HMW2) (11), H. influenzae adhesin (12), Haemophilus adhesion and penetration (Hap) protein (13), OMP P5 (14), and the opacity-associated (Opa) protein A (15).
Bacterial colonization may induce host damage and trigger specific immune responses that can eradicate the bacteria. T lymphocytes detect microbial antigens in conjunction with major histocompatibility complex molecules. In the presence of costimulatory signals, T cells become activated and produce cytokines that regulate immune responses. T helper-1 (Th1) cells are generated by T-bet expression through STAT1 and STAT4 upon T cell receptor (TCR) stimulation in the presence of interferon-gamma (IFN-γ) and interleukin-12 (IL-12), respectively. T helper-2 cells are stimulated by activation of TCR-stimulated T cell factor 1 and IL-2 and IL-4 signaling (16).
Mucosal CD4+ T cell priming occurs in mucosa-associated lymphoid tissue following bacterial invasion, leading to the production of effector and memory T cells (17). Activated CD4+ T cells produce both Th1 cytokines, such as TNF-α and IFN-γ, and Th2 cytokines, including IL-5 and IL-10. Th1 cytokines activate cell-mediated immunity, whereas Th2 cytokines stimulate humoral immunity (18,19). The sequel of infectious diseases is largely dependent on the balance between Th1 and Th2 cytokines (20).
Bacteria can inhibit lymphocyte proliferation and cytokine production, while others can stimulate the production of pro-inflammatory cytokines. Failure to evoke a strong proinflammatory response from immune cells might impair host clearance of pathogens and prolong colonization. Haemophilus influenzae utilizes capsular polysaccharide for immune evasion, resulting in a deficiency of co-stimulatory signals that impairs robust lymphocyte activation and proliferation (21). Also, it can directly trigger programmed cell death in lymphocytes, effectively shutting down the adaptive immune response before it can fully develop (22).
Previous studies highlighted the importance of lymphocyte responses in the pathogenesis of NTHi infection. The incidence of COPD exacerbations due to NTHi suggests that the immunological defense mechanisms against these bacteria are hindered. It has been demonstrated that exacerbations of COPD are associated with a decrease in the proliferation of T cells in response to the outer membrane lipoprotein P6 of NTHi (23). Additionally, CD4+ memory T cells specific for NTHi were present at low rates in the peripheral blood of COPD patients and healthy controls (24). Furthermore, COPD due to NTHi was associated with Th2 cytokines and decreased expression of CD40 ligand (1). However, the immune responses of CD4+ T cells to H. influenzae are not clearly characterized. This study aimed to investigate the effects of live variants of single NTHi and THi strains on CD4+ T cell proliferation and production of Th1 and Th2 cytokines in vitro.
Materials and Methods
Bacterial Strains
Variants of a single strains of THi (132b+, 132b-, 132b-p5-) and NTHi (A950002, A950002p5-, A850052, d1, d3) were used in this study. Bacterial strains 132b-, A950002, and d1 express P5 protein whereas P5 deficient strains include 132b-p5-, A950002p5-, and d3. Haemophilus influenzae strains were cultivated on brain heart infusion agar enriched with 5% blood.
Isolation of Primary CD4+ T Cells
Peripheral blood (18 mL) was collected from healthy individuals and placed in a Falcon tube containing 2 mL sodium citrate as an anticoagulant. The blood was carefully layered over 20 mL of Histopaque®-1077 (Sigma-Aldrich, Dorset, UK) in 50 mL conical tubes. Following centrifugation, peripheral blood mononuclear cells (PBMCs) were extracted from the buffy coat layer. CD4+ T cells were then isolated from the PBMCs using negative selection, adhering to the protocol provided with the immunomagnetic CD4+ T cell isolation kit (Miltenyi Biotec, Surrey, UK). The cells were subsequently cultured in RPMI 1640 medium (Sigma Aldrich, Dorset, UK), enriched with 10% inactivated fetal bovine serum, 20 mM HEPES buffer (Sigma-Aldrich, Dorset, UK), 1% L-glutamine (Sigma-Aldrich, Dorset, UK). Cell culture was kept in a humidified incubator at 37°C with 5% CO2. For adhesion, cell proliferation, and cytokines assays, the T cells were infected with live strains of H. influenzae at a multiplicity of infection (MOI) of 100:1. This MOI was chosen based on a previously published study (25).
Bacterial Adhesion Assay
To upregulate carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) on the surface of CD4+ T cells, IL-2 (200 U/mL) was added to the cell cultures for 48 hours. After washing, the cells were infected with live strains of THi and NTHi at MOI of 100:1 and incubated for 3 hours at 37°C in a humidified atmosphere of 5% CO2. Cells and bacteria were transferred to a 5 μm Transwell filter (Costar, Corning, NY, USA). To remove nonadherent bacteria, the filters were washed six times with Hanks' balanced salt solution (HBSS; Sigma-Aldrich, Dorset, UK). The filters were then transferred to new wells, and 200 μL of 1% saponin was added per filter for 30 minutes to release cell-associated bacteria. These bacteria were harvested by four 200 μL washes with HBSS, and suspensions of bacteria were plated after appropriate dilutions to estimate the colony-forming units (CFU). The bacterial adhesion assay was conducted in duplicate, and adhesion was quantified by counting the number of bacterial CFUs attached to the CD4+ T cells, as previously described (25).
T Cell Proliferation Assay and Th1/Th2 Cytokine Analysis
To evaluate the impact of live bacteria on CD4+ T cell proliferation, cells were seeded at a density of 2 × 105 cells/well in 24-well plates pre-coated with anti-CD3 antibody (OKT3) at 1 µg/mL. Simultaneously, the cells were infected with bacteria at MOI of 100:1. Gentamicin (50 µg/mL) was added 3 hours post-infection and maintained throughout the experiment to stop bacterial overgrowth. On day 3, cell proliferation was evaluated by determining the [³H]-thymidine incorporation, as outlined in prior studies (26). Briefly, 100 µL aliquots of each CD4+ T cell culture were added in triplicate to a 96-well plate, pulsed with [³H]-thymidine (1 µCi/well), and incubated for 6 hours in a humidified atmosphere of 5% CO2 at 37°C. Radioactivity was quantified by a liquid scintillation beta counter (1450 Microbeta; LKB Wallac, Turku, Finland). The results were expressed as the average counts per minute (CPM) of duplicate cultures. Additionally, 50 µL samples of cell supernatants were collected in duplicate on day 3 for cytokine analysis. The levels of IFN-γ, TNF-α, IL-5, and IL-10 were measured using a human Th1/Th2 cytokine cytometric bead array (CBA) kit (BD Biosciences, Oxford, UK), following the manufacturer’s guidelines. Cytokine concentrations were determined based on fluorescent intensities (FL2), and average values were computed using CBA software (BD Biosciences, Oxford, UK). The Th1/Th2 CBA assay is a reliable and sensitive method for simultaneously quantifying multiple cytokines (27).
Statistical Analysis
All data were analyzed using GraphPad Prism version 8 (GraphPad Software, San Diego, CA, USA). Results are presented as mean ± standard error of the mean (SEM). Statistical comparisons were performed using one-way analysis of variance (ANOVA) with Tukey's and Dunnett's multiple comparison tests, and unpaired t-tests. The statistical significance was set as p<0.05. In figures, significance is indicated as follows: * = p≤0.05, ** = p≤0.01, *** = p ≤0.001, **** = p≤ 0.0001, ns = p>0.05.
Results
Adhesion of Haemophilus influenzae to CD4+ T Cells
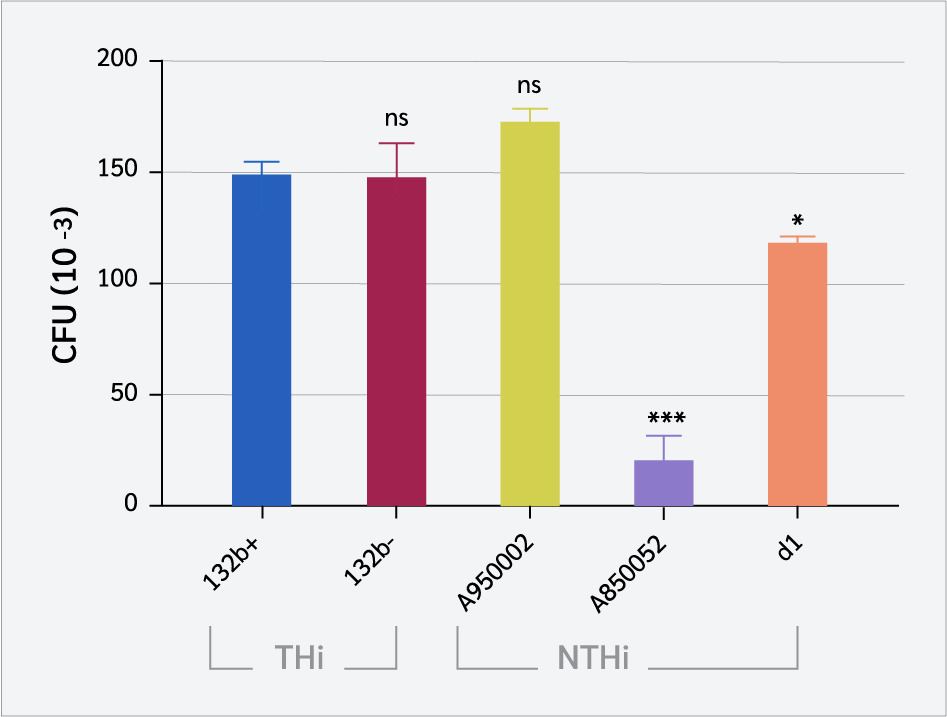
Variants of single strains of THi and NTHi were used to assess the adhesion of H. influenzae to CD4+ T cells. Carcinoembryonic antigen-related cell adhesion molecule 1 was upregulated on CD4+ T cells by stimulation with IL-2 before infection with bacteria. As shown in Figure 1, both THi and NTHi strains are bound to CD4+ T cells to varying degrees. Typeable H. influenzae (132b+ and 132b-) bind more to CD4+ T cells compared to NTHi A850052 (p=0.0004) and d1 (p=0.0196 and 0.0219), respectively. However, there was no significant difference between THi (132b+ or 132b-) and NTHi A950002 (p=0.3812 and 0.3325), respectively.
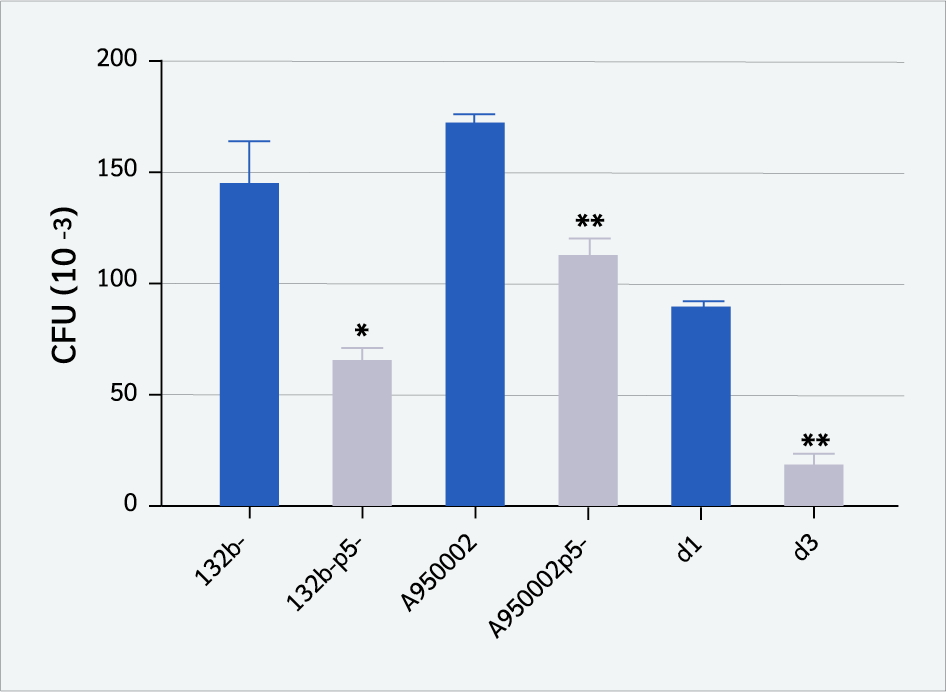
As shown in Figure 2, the presence of P5 protein in P5+ strains (132b-, A950002, and d1) increased the binding to CD4+ T cells significantly compared to P5-deficient strains (132b-p5-, A950002p5-, and d3), with p value less than 0.05 [132b- vs. 132b-p5- (p=0.0009), A950002 vs. A950002p5- (p=0.0039), d1 vs. d3 (p=0.0014)]. However, P5-deficient strains can still bind to T cells. These findings suggest that the P5 protein contributes to H. influenzae binding to T cells in a significant but non-essential way.
Effect of Haemophilus influenzae on CD4+ T Cell Proliferation
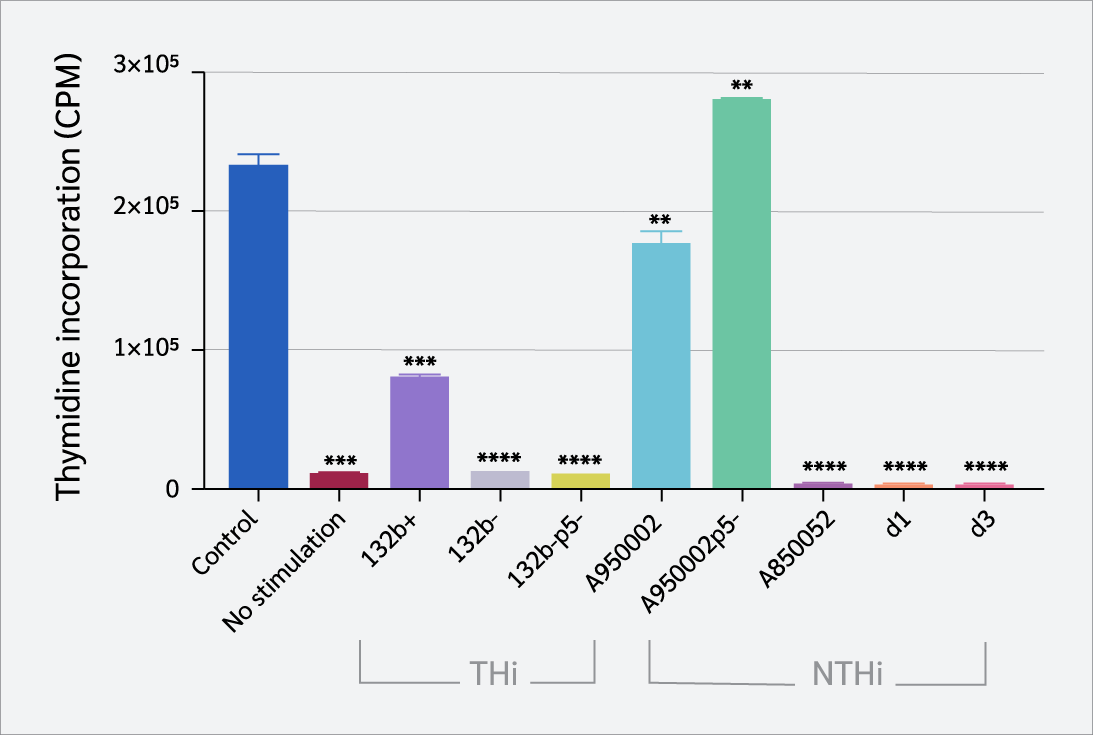
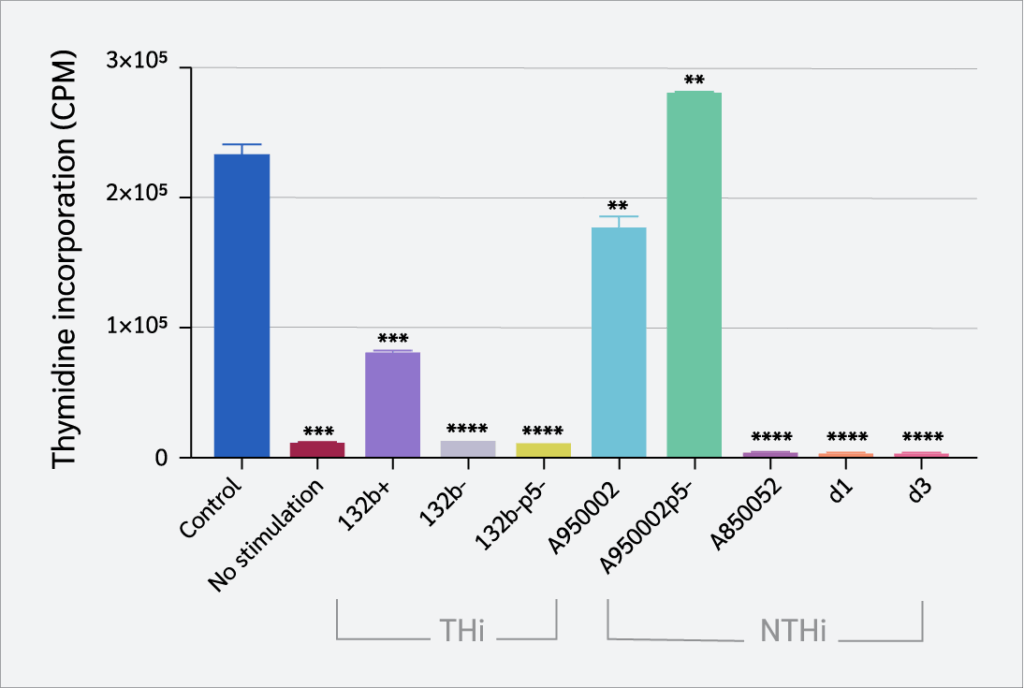
CD4+ T cells were stimulated by immobilized anti-CD3 (iCD3) antibody. The cells were infected with live THi or NTHi strains in the presence of iCD3 antibody. Figure 3 shows that THi (132b-, 132b-p5) and NTHi strains (A850052, d1, d3) caused marked inhibition of CD4+ T cell proliferation (p<0.0001), whereas THi strain 132b+ and NTHi strain A950002 induced moderate inhibition compared to uninfected controls (p=0.0004, p=0.0079), respectively. In contrast, NTHi strain A950002p5- increased proliferation slightly (p=0.0064). The overall pattern of CD4+ T cell proliferation was not significantly influenced by the P5 protein.
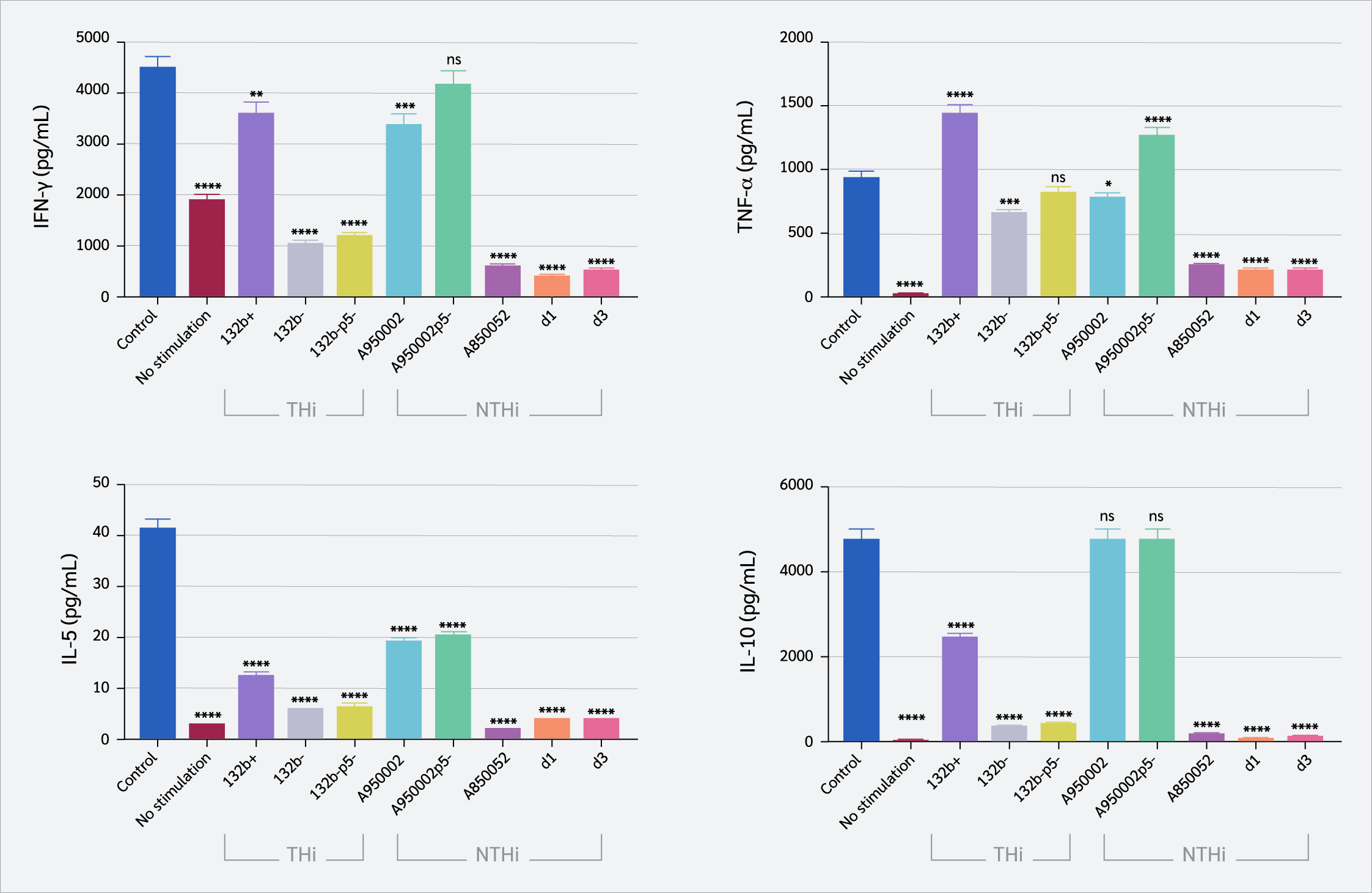
Effect of Haemophilus influenzae on Th1 and Th2 Cytokine Production
The production of Th1 (IFN-γ and TNF-α) and Th2 (IL-5, and IL-10) cytokines by CD4+ T cells were assessed using a cytometric bead array to assess Th1 and Th2 immunological responses to H. influenzae infection (Figure 4). IFN-γ production was significantly inhibited by both THi and NTHi strains (except A950002p5- strain), with marked suppression observed for NTHi strains (A850052, d1, d3) and the THi strains 132b- and 132b-p5- (p<0.0001). TNF-α production was significantly inhibited by132b- strain (p=0.0002), A950002 strain (p=0.01), A850052, d1and d3 strains (p<0.0001). In contrast, THi strain 132b+ and NTHi strain A950002p5 significantly increased TNF-α production (p<0.0001). IL-5 production was reduced by all THi and NTHi strains (p<0.0001), while all THi and NTHi (A850052, d1, d3) decreased IL-10 production (p<0.0001).
Discussion
This study demonstrates that both THi and NTHi strains of H. influenzae bind to CD4+ T cells at varying degrees. The presence of the P5 protein enhanced binding in both strain types; however, they can still bind to T cells in its absence, likely due to the presence of other adhesins on the bacterial surface. Activated human CD4+ T cells express CEACAM1 (28), β1 integrins (29), and Toll-like receptor (TLR) 2 and TLR4 (30). It has been demonstrated that the OMP-P2 and P6 interact with TLR2 (22,31,32), and the P5 protein interacts with CEACAM1 (33,34). Additionally, NTHi strain d1 expresses P5 protein bound strongly to Chinese hamster ovary (CHO)-CEACAM1 cells, whereas P5-deficient d3 strain did not (34). Moreover, H. influenzae expresses a range of adhesins that bind to host cells, such as Haemophilus surface fibrils that bind to vitronectin (35), and pili, which adhere to fibronectin and heparin-binding matrix proteins (36,37).
Immunity to microbial infection is organized by Th cells. Th1 cells are essential for immunity to intracellular pathogens via the production of IFN-γ, which activates macrophages, and IL-2, which induces lymphocyte proliferation. On the contrary, Th2 cells produce IL-4, IL-5, IL-10, and IL-13, which support humoral immunity and play a crucial role in eliminating extracellular pathogens (38). Antimicrobial Th1 and Th2 responses are typically linked to resistance and susceptibility to infectious diseases, respectively. This was demonstrated in intracellular pathogens Leishmania major and Mycobacterium leprae (18,39). The interaction of H. influenzae with mucosal CD4+ T cells is a significant factor in determining the outcome of H. influenzae infection. Herein, the results of this study have shown that certain live strains of NTHi and THi inhibited T cell proliferation and markedly reduced Th1 and Th2 cytokine production. This immunosuppressive effect may represent a mechanism by which the adaptive immune response is evaded.
Bacteria can manipulate the inhibitory signaling to avoid host defense. IgA proteases and phase variation of lipopolysaccharide enable NTHI to evade mucosal immune mechanisms and invade respiratory epithelial cells, allowing it to live intracellularly (3). On the contrary, H. influenzae can stimulate CD4+ T cell immune responses (1). In response to the NTHi antigen, the activated Th (CD4+CD69+) cells produced Th1 cytokines (IFN-γ and IL-2) in healthy controls (1). However, the bronchiectasis group had predominant Th2 cytokines such as IL-4 and IL-10 (1). In murine models, nasal immunization with NTHi antigen induced specific Th1 and Th2 responses (40). Furthermore, stimulation with P6 protein induced CD4+ T cell proliferation in P6-immunized mice, and these cells upregulated mRNA for Th2 cytokines (41). The findings presented in this study may appear to be different from previous reports. Unlike prior studies that used bacterial antigens (1,40,41). The bacterial strains used in this study are well-characterized, specific live strains, which may explain the difference.
The inhibition of T-cell proliferation can be explained by several mechanisms. Haemophilus ducreyi-reactive CD4+ T cell proliferation was markedly increased by CD25+CD4+ T cell depletion, underscoring the function of regulatory T (Treg) cells in regulating the immunological response to bacterial infection (42). Regulatory T cells can suppress the immune responses through cellular interactions and/or release of IL-10 and TGF-β (43). It has been shown that IL-10 released by activated B lymphocytes in humans inhibited CD4+CD25- T-cell proliferation in vitro (44). Furthermore, Foxp3+ CD25+ Treg cells may produce suppressor cytokines, such as TGF-β and IL-10, induce apoptosis or granzyme-mediated cytolysis, or compete with effector T cells for IL-2. After Treg activation, the cells may express galectin-1, which may arrest the cell cycle when interacting with receptors on effector T cells (45).
This study demonstrated the immunosuppressive properties of certain H. influenzae strains in vitro.
However, it has some limitations that may affect its relevance to in vivo settings. One of the limitations is the lack of systemic immune components, such as circulating cytokines and complement proteins, which modulate immune responses in vivo. In addition, using isolated immune cell types rather than mixed populations found in vivo results in the absence of immune cell crosstalk and an incomplete immune response.Conclusion
The present study demonstrates that certain strains of H. influenzae inhibit the proliferation of primary human CD4+ T cells and suppress the production of Th1 and Th2 cytokines. This immunosuppressive effect may contribute to the ability of H. influenzae to evade the adaptive immune response and establish respiratory colonization and persistence of the infection. The molecular mechanisms behind H. influenzae's immunosuppressive effect on CD4+ T cells require more research.
Ethical Approval
The study was approved by the Biomedical Research Ethics Committee of Umm Al-Qura University on December 25, 2024, with the decision number VDJB171224.
Informed Consent
N.A.
Peer-review
Externally peer-reviewed
Author Contributions
Concept – A.R.Y.; Design – A.R.Y.; Supervision – A.R.Y.; Data Collection and/or Processing – A.R.Y.; Analysis and/or Interpretation – A.R.Y.; Literature Review – A.R.Y.; Writer – A.R.Y.; Critical Reviews – A.R.Y.
Conflict of Interest
The author declares no conflict of interest.
Financial Disclosure
The author declared that this study has received no financial support.
Acknowledgment
I am grateful to Professor Mumtaz Virji for scientific support and to Dr. Andrew Herman for assistance with flow cytometry.
References
King PT, Hutchinson PE, Johnson PD, Holmes PW, Freezer NJ, Holdsworth SR. Adaptive immunity to nontypeable Haemophilus influenzae. Am J Respir Crit Care Med. 2003;167(4):587-92. [CrossRef]
Turk DC. The pathogenicity of Haemophilus influenzae. J Med Microbiol. 1984;18(1):1-16. [CrossRef]
Zhang J, Zhu Z, Zuo X, Pan H, Gu Y, Yuan Y, et al. The role of NTHi colonization and infection in the pathogenesis of neutrophilic asthma. Respir Res. 2020;21(1):170. [CrossRef]
Broome CV. Epidemiology of Haemophilus influenzae type b infections in the United States. Pediatr Infect Dis J. 1987;6(8):779-82. [CrossRef]
Murphy TF, Sethi S, Klingman KL, Brueggemann AB, Doern GV. Simultaneous respiratory tract colonization by multiple strains of nontypeable Haemophilus influenzae in chronic obstructive pulmonary disease: implications for antibiotic therapy. J Infect Dis. 1999;180(2):404-9. [CrossRef]
Erwin AL, Smith AL. Nontypeable Haemophilus influenzae: understanding virulence and commensal behavior. Trends Microbiol. 2007;15(8):355-62. [CrossRef]
Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359(22):2355-65. [CrossRef]
Ganesan S, Comstock AT, Kinker B, Mancuso P, Beck JM, Sajjan US. Combined exposure to cigarette smoke and nontypeable Haemophilus influenzae drives development of a COPD phenotype in mice. Respir Res. 2014;15(1):11. [CrossRef]
Weeks JR, Staples KJ, Spalluto CM, Watson A, Wilkinson TMA. The role of non-typeable Haemophilus influenzae biofilms in chronic obstructive pulmonary disease. Front Cell Infect Microbiol. 2021;11:720742. [CrossRef]
Gilsdorf JR, McCrea KW, Marrs CF. Role of pili in Haemophilus influenzae adherence and colonization. Infect Immun. 1997;65(8):2997-3002. [CrossRef]
Bakaletz LO, Barenkamp SJ. Localization of high-molecular-weight adhesion proteins of nontypeable Haemophilus influenzae by immunoelectron microscopy. Infect Immun. 1994;62(10):4460-8. [CrossRef]
Barenkamp SJ, St Geme JW 3rd. Identification of a second family of high-molecular-weight adhesion proteins expressed by non-typable Haemophilus influenzae. Mol Microbiol. 1996;19(6):1215-23. [CrossRef]
St Geme JW 3rd, de la Morena ML, Falkow S. A Haemophilus influenzae IgA protease-like protein promotes intimate interaction with human epithelial cells. Mol Microbiol. 1994;14(2):217-33. [CrossRef]
Bookwalter JE, Jurcisek JA, Gray-Owen SD, Fernandez S, McGillivary G, Bakaletz LO. A carcinoembryonic antigen-related cell adhesion molecule 1 homologue plays a pivotal role in nontypeable Haemophilus influenzae colonization of the chinchilla nasopharynx via the outer membrane protein P5-homologous adhesin. Infect Immun. 2008;76(1):48-55. [CrossRef]
Prasadarao NV, Lysenko E, Wass CA, Kim KS, Weiser JN. Opacity-associated protein A contributes to the binding of Haemophilus influenzae to chang epithelial cells. Infect Immun. 1999;67(8):4153-60. [CrossRef]
Sun L, Su Y, Jiao A, Wang X, Zhang B. T cells in health and disease. Signal Transduct Target Ther. 2023;8(1):235. [CrossRef]
Ebert LM, Schaerli P, Moser B. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol Immunol. 2005;42(7):799-809. [CrossRef]
Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383(6603):787-93. [CrossRef]
O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8(3):275-83. [CrossRef]
Spellberg B, Edwards JE Jr. Type 1/Type 2 immunity in infectious diseases. Clin Infect Dis. 2001;32(1):76-102. [CrossRef]
Moxon ER, Kroll JS. The role of bacterial polysaccharide capsules as virulence factors. Curr Top Microbiol Immunol. 1990;150:65-85. [CrossRef]
Galdiero M, Galdiero M, Finamore E, Rossano F, Gambuzza M, Catania MR, et al. Haemophilus influenzae porin induces Toll-like receptor 2-mediated cytokine production in human monocytes and mouse macrophages. Infect Immun. 2004;72(2):1204-9. [CrossRef]
Abe Y, Murphy TF, Sethi S, Faden HS, Dmochowski J, Harabuchi Y, et al. Lymphocyte proliferative response to P6 of Haemophilus influenzae is associated with relative protection from exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;165(7):967-71. [CrossRef]
de Bree GJ, Daniels H, Schilfgaarde Mv, Jansen HM, Out TA, van Lier RA, et al. Characterization of CD4+ memory T cell responses directed against common respiratory pathogens in peripheral blood and lung. J Infect Dis. 2007;195(11):1718-25. [CrossRef]
Youssef AR, van der Flier M, Estevão S, Hartwig NG, van der Ley P, Virji M. Opa+ and Opa- isolates of Neisseria meningitidis and Neisseria gonorrhoeae induce sustained proliferative responses in human CD4+ T cells. Infect Immun. 2009;77(11):5170-80. [CrossRef]
Youssef AR, Elson CJ. Induction of IL-10 cytokine and the suppression of T cell proliferation by specific peptides from red cell band 3 and in vivo effects of these peptides on autoimmune hemolytic anemia in NZB mice. Auto Immun Highlights. 2017;8(1):7. [CrossRef]
Morgan E, Varro R, Sepulveda H, Ember JA, Apgar J, Wilson J, et al. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin Immunol. 2004;110(3):252-66. [CrossRef]
Moller MJ, Kammerer R, Grunert F, von Kleist S. Biliary glycoprotein (BGP) expression on T cells and on a natural-killer-cell sub-population. Int J Cancer. 1996;65(6):740-5. [CrossRef]
Woods ML, Shimizu Y. Signaling networks regulating beta1 integrin-mediated adhesion of T lymphocytes to extracellular matrix. J Leukoc Biol. 2001;69(6):874-80.
Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci U S A. 2004;101(9):3029-34. [CrossRef]
Chen R, Lim JH, Jono H, Gu XX, Kim YS, Basbaum CB, et al. Nontypeable Haemophilus influenzae lipoprotein P6 induces MUC5AC mucin transcription via TLR2-TAK1-dependent p38 MAPK-AP1 and IKKbeta-IkappaBalpha-NF-kappaB signaling pathways. Biochem Biophys Res Commun. 2004;324(3):1087-94. [CrossRef]
Berenson CS, Murphy TF, Wrona CT, Sethi S. Outer membrane protein P6 of nontypeable Haemophilus influenzae is a potent and selective inducer of human macrophage proinflammatory cytokines. Infect Immun. 2005;73(5):2728-35. [CrossRef]
Muenzner P, Rohde M, Kneitz S, Hauck CR. CEACAM engagement by human pathogens enhances cell adhesion and counteracts bacteria-induced detachment of epithelial cells. J Cell Biol. 2005;170(5):825-36. [CrossRef]
Hill DJ, Toleman MA, Evans DJ, Villullas S, Van Alphen L, Virji M. The variable P5 proteins of typeable and non-typeable Haemophilus influenzae target human CEACAM1. Mol Microbiol. 2001;39(4):850-62. [CrossRef]
Hallström T, Trajkovska E, Forsgren A, Riesbeck K. Haemophilus influenzae surface fibrils contribute to serum resistance by interacting with vitronectin. J Immunol. 2006;177(1):430-6. Erratum in: J Immunol. 2013;190(8):4431. [CrossRef]
Bresser P, Virkola R, Jonsson-Vihanne M, Jansen HM, Korhonen TK, van Alphen L. Interaction of clinical isolates of nonencapsulated Haemophilus influenzae with mammalian extracellular matrix proteins. FEMS Immunol Med Microbiol. 2000;28(2):129-32. [CrossRef]
Virkola R, Brummer M, Rauvala H, van Alphen L, Korhonen TK. Interaction of fimbriae of Haemophilus influenzae type B with heparin-binding extracellular matrix proteins. Infect Immun. 2000;68(10):5696-701. [CrossRef]
Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145-73. [CrossRef]
Reiner SL, Locksley RM. The regulation of immunity to leishmania major. Annu Rev Immunol. 1995;13:151-77. [CrossRef]
Kurono Y, Yamamoto M, Fujihashi K, Kodama S, Suzuki M, Mogi G, et al. Nasal immunization induces Haemophilus influenzae-specific Th1 and Th2 responses with mucosal IgA and systemic IgG antibodies for protective immunity. J Infect Dis. 1999;180(1):122-32. [CrossRef]
Kodama S, Suenaga S, Hirano T, Suzuki M, Mogi G. Induction of specific immunoglobulin A and Th2 immune responses to P6 outer membrane protein of nontypeable Haemophilus influenzae in middle ear mucosa by intranasal immunization. Infect Immun. 2000;68(4):2294-300. [CrossRef]
Li W, Tenner-Racz K, Racz P, Janowicz DM, Fortney KR, Katz BP, et al. Role played by CD4+FOXP3+ regulatory T Cells in suppression of host responses to Haemophilus ducreyi during experimental infection of human volunteers. J Infect Dis. 2010;201(12):1839-48. [CrossRef]
Roncarolo MG, Gregori S, Levings M. Type 1 T regulatory cells and their relationship with CD4+CD25+ T regulatory cells. Novartis Found Symp. 2003;252:115-27.
Bouaziz JD, Calbo S, Maho-Vaillant M, Saussine A, Bagot M, Bensussan A, et al. IL-10 produced by activated human B cells regulates CD4(+) T-cell activation in vitro. Eur J Immunol. 2010;40(10):2686-91. [CrossRef]
Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30(5):636-45. [CrossRef]
VOLUME
,
ISSUE
Correspondence
Received
Accepted
Published
Suggested Citation
DOI
License